|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive Nov 10, 2014
Structures of the hypothalamus and the pituitary gland in the human brain produce these hormones.
|
|
|
|
|
|
Sugars help us differentiate seasons
Scientists have discovered how a single hormone manages to trigger two different physiologic functions — the sensing of season changes and functional metabolism, without any mixup.
Extensive national and international collaboration between ITbM, Nagoya University, and the University of Chicago has uncovered how thyrotropin, a thyroid stimulating hormone (TSH), triggers two different functions when released into the bloodstream, yet manages to avoid crosstalk.
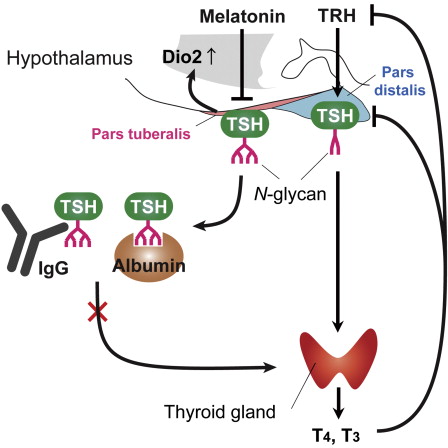
TSH is a glycoprotein — a protein that contains carbohydrate — and is secreted from two parts of the brain's pituitary gland: the stalk of the pituitary gland called the pars tuberalis; and from the front of the pituitary gland called the pars distalis.
Previous studies have shown that TSHs secreted from both of these regions have the same protein structure. However, TSH secreted from the pars distalis stimulates thyroid to secrete thyroid hormones regulating metabolism and growth, while Yoshimura's group found that TSH secreted from the pars tuberalis acts on the hypothalamus to send information on seasonal change.
It had been a mystery how these two TSHs manage to distinctively trigger biologically significant processes without interfering with one another. However, a study published on October 30, 2014 in Cell Reports, revealed that the same molecule imparts different functions without cross activity by using tissue-specific glycosylation — attaching sugars — which assist recognition by the immune system. This new paradigm is expected to contribute towards our understanding of diseases related to the synthesis and secretion of TSHs.
Many organisms adapt to seasonal changes by detecting changes in the amount of sunlight.
Observed with mass spectrometry analysis, both PT-TSH and PD-TSH share the same protein structure. But each has a different carbohydrate chain attached that distinguishes them: PD-TSH has sulfated bi-antennary carbohydrate chains, which are easily metabolized; and PT-TSH has sialylated multi-branched carbohydrate chains, that stabilize macro-TSH complexes and therefore do not stimulate the thyroid.
Although the fundamental importance of glycosylation has been recognized in recent years, its physiologic function had remained unclear. This study illustrates the first tissue-specific glycosylation that prevents crosstalk between signaling molecules.
Prof. Yoshimura : "As the genome is finite, organisms use the same protein to serve multiple functions. Through our research, we were able to uncover the elegant strategy of tissue-specific glycosylation to impart 2 distinctive functions on a single hormone."
Highlights
•Tissue-specific glycosylation imparts specificity to thyroid-stimulating hormone
•Pars tuberalis-TSH (PT-TSH) does not stimulate the thyroid gland
•PT-TSH has tissue-specific N-glycans and forms macro-TSH in blood
•Macro-TSH circulating in blood does not stimulate thyroid hormone secretion
Summary
Thyroid-stimulating hormone (TSH; thyrotropin) is a glycoprotein secreted from the pituitary gland. Pars distalis-derived TSH (PD-TSH) stimulates the thyroid gland to produce thyroid hormones (THs), whereas pars tuberalis-derived TSH (PT-TSH) acts on the hypothalamus to regulate seasonal physiology and behavior. However, it had not been clear how these two TSHs avoid functional crosstalk. Here, we show that this regulation is mediated by tissue-specific glycosylation. Although PT-TSH is released into the circulation, it does not stimulate the thyroid gland. PD-TSH is known to have sulfated biantennary N-glycans, and sulfated TSH is rapidly metabolized in the liver. In contrast, PT-TSH has sialylated multibranched N-glycans; in the circulation, it forms the macro-TSH complex with immunoglobulin or albumin, resulting in the loss of its bioactivity. Glycosylation is fundamental to a wide range of biological processes. This report demonstrates its involvement in preventing functional crosstalk of signaling molecules in the body.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Professor Takashi Yoshimura and Dr. Keisuke Ikegami (currently at Kinki University) at the Institute of Transformative Bio-Molecules (ITbM) of Nagoya University, Prof. Samuel Refetoff of the University of Chicago
Authors
This article "Tissue-specific post-translational modification allows functional targeting of thyrotropin" by Keisuke Ikegami, Xiao-Hui Liao, Yuta Hoshino, Hiroko Ono, Wataru Ota, Yuka Ito, Taeko Nishiwaki-Ohkawa, Chihiro Sato, Ken Kitajima, Masayuki Iigo, Yasufumi Shigeyoshi, Masanobu Yamada, Yoshiharu Murata, Samuel Refetoff, Takashi Yoshimura is published online on October 30, 2014 in Cell Reports.
DOI: 10.1016/j.celrep.2014.10.006
About WPI-ITbM
The World Premier International Research Center Initiative (WPI) for the Institute of Transformative Bio-Molecules (ITbM) at Nagoya University in Japan is committed to advance the integration of synthetic chemistry, plant/animal biology and theoretical science, all of which are traditionally strong fields in the university. As part of the Japanese science ministry's MEXT program, ITbM aims to develop transformative bio-molecules, innovative functional molecules capable of bringing about fundamental change to biological science and technology. Research at ITbM is carried out in a "Mix-Lab" style, where international young researchers from multidisciplinary fields work together side-by-side in the same lab. Through these endeavors, ITbM will create "transformative bio-molecules" that will dramatically change the way of research in chemistry, biology and other related fields to solve urgent problems, such as environmental issues, food production and medical technology that have a significant impact on society.
Return to top of page
|
