|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Developmental biology - Maternal Care Mother's attention changes baby's genes New evidence suggests that the amount of licking and grooming from a mother mouse to her pups can alter the function of her pup's genes. Transposons or transposable elements can cause deletions or inversions in a DNA strand affecting how a gene functions. When transposition generates two copies of the same sequence in the same orientation (direction), recombination can delete the DNA which had existed between them. Retrotransposons also move by a "copy and paste" mechanism — but in contrast to the transposons described above, the copy is made of RNA, not DNA. The RNA copies are then transcribed back into DNA — using a reverse transcriptase — and these are inserted into new locations in the genome. Tracy Bedrosian PhD, and colleagues sought to explore the influence of maternal care on the prevalence of pups' transposons which are the genetic sequences that can spontaneously replicate themselves throughout a genome (meaning a body's entire collection of genes). In particular, they studied L1 retrotransposons, of which the mouse genome has more than 3,000. Mother mice and their newborn pups were monitored for two weeks, then assigned to two different groups based on low and high levels of maternal care. Pups that received less maternal care exhibited noticeably more transposon replicated in their hippocampus. 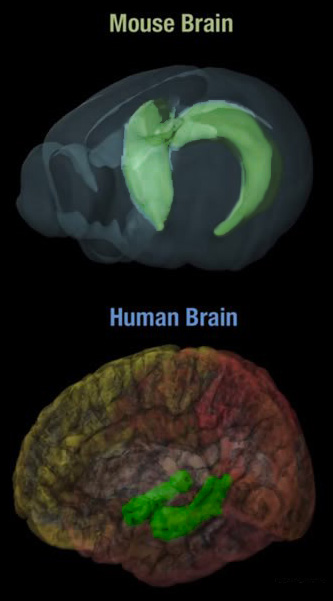 Top: Mouse hippocampus; Bottom: Human hippocampus Intriguingly, the effect was not observed in the frontal cortex of the brain or in the heart. The work, appearing in the journal Science, suggests that it is unlikely to be the result of inherited differences in the numbers of L1 copies. Mother mice and their newborn pups were monitored for two weeks, then assigned to two different groups based on low and high levels of maternal care. Pups that received less maternal care exhibited noticeably more transposon replication in the hippocampus, the authors report. Intriguingly, this effect was not observed in the frontal cortex or heart, hinting that it is unlikely to be a result of inherited differences in the numbers of L1 copies. As well, experiments where pups were raised by foster mothers support the influence of maternal care, rather than genetic makeup. Lastly, Bedrosian and her group point to a recent report that childhood stress and adversity result in hypomethylation of retrotransposons in humans, noting however that the average human genome has many fewer (about 100) active retrotransposon copies than the mouse genome with its 3,000. Genomic plasticity during brain development Mice genomes contain many mobile retrotransposons. Bedrosian et al. analyzed DNA from the mouse hippocampus during development (see the Perspective by Song and Gleeson). They found that the amount of maternal care in the first few weeks of a mouse pup's life affected the number of copies of the L1 retrotransposon. The experience of maternal care was thus “recorded” in the DNA of these mice pups during a time when the brain was still actively developing. Abstract The brain is a genomic mosaic owing to somatic mutations that arise throughout development. Mobile genetic elements, including retrotransposons, are one source of somatic mosaicism in the brain. Retrotransposition may represent a form of plasticity in response to experience. Here, we use droplet digital polymerase chain reaction to show that natural variations in maternal care mediate the mobilization of long interspersed nuclear element–1 (LINE-1 or L1) retrotransposons in the hippocampus of the mouse brain. Increasing the amount of maternal care blocks the accumulation of L1. Maternal care also alters DNA methylation at YY1 binding sites implicated in L1 activation and affects expression of the de novo methyltransferase DNMT3a. Our observations indicate that early life experience drives somatic variation in the genome via L1 retrotransposons. Summary The brain is constantly changing in response to environmental experiences throughout life. Mounting evidence from animal and human studies suggests that brain development and behavior are influenced by early life experiences. Several compelling experimental models have been developed to study the effect of early life experiences on the brain, such as stress, exposure to toxins, availability of nutrients, adversity, and quality of maternal care (1). The relationship between genes and environment on the brain and how they affect behavior has been a long-standing issue. Can the genome of individual brain cells be changed by environmental factors? If so, which types of genetic changes can result? What is the molecular basis of this genetic diversity? What are the physiological implications? On page 1395 of this issue, Bedrosian et al. (2) explore one possibility for how neuronal genomes can exhibit plasticity in response to environmental factors during early life, providing integrative evidence for the effect of early maternal care on the genomes of neurons. Authors: Tracy A. Bedrosian, Carolina Quayle, Nicole Novaresi, Fred. H. Gage. Return to top of page | Mar 26, 2018 Fetal Timeline Maternal Timeline News News Archive  A new study in mice reveals that pups receiving less maternal care have more repeated genetic sequences, called transposons, in neurons that reside in their hippocampus. The implications of this phenomenon are unknown, so further research is needed. Image: Tom McHugh Science Source Images.
|
||||||||||||||||||||||||||||

