|
|
Developmental Biology - DMG Gliomas
New Attack Plan Against DMG Gliomas
Promising drug combination against lethal childhood brain cancers...
Studies in cell and animal models reveal insights into cancer cells' vulnerability that could lead to new strategies against brain cancers.
Researchers devised a new plan of attack against a group of deadly childhood brain cancers collectively called diffuse midline gliomas (DMG), including (1) diffuse intrinsic pontine glioma (DIPG), (2) thalamic glioma (3) spinal cord glioma.
Scientists at the National Institutes of Health, Stanford University, California, and Dana-Farber Cancer Institute, Boston, have identified a drug pair that works together to both kill cancer cells and counter effects of a genetic mutation that causes these diseases.
Research shows that combining two drugs - panobinostat and marizomib - is more effective than either drug by itself in killing DMG cells grown from patients in the laboratory and then in animal models. They also uncovered a previously unrecognized vulnerability in these cancer cells that scientists may be able to develop into new strategies against these cancer and related diseases.
Their results are published in Science Translational Medicine.
Matrix Screening Delivers Insights, increases options
DMGs are aggressive, hard-to-treat tumors representing the leading cause of brain cancer-related death among children in the USA. They typically affect a few hundred children a year between ages 4 to 12 — with most dieing within a year of diagnosis.
They are most often caused by a specific mutation in histone genes, protein complexes located in the cell nucleus. DNA wraps around a histone to form chromatin, which condenses DNA to fit within the nucleus. Enzymes determine how DNA winds and unwinds around histones, including histone deacetylases (enzymes). These enzymes add or remove chemical tags that indirectly control if genes are turned on or off.
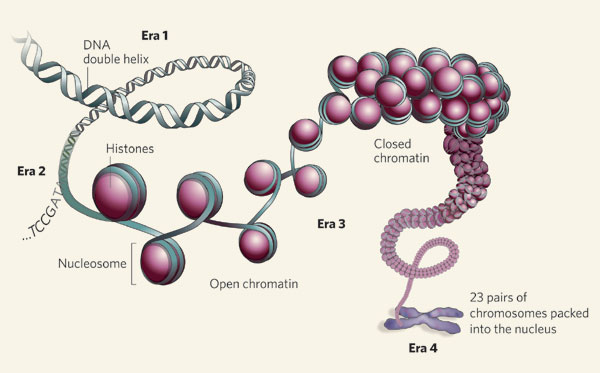
A nucleosome incoporates DNA, histone core and histone. Credit: Nature Genomic architecture.
In an earlier study, Stanford neuro-oncologist Michelle Monje MD PhD, and her colleagues showed how panobinostat, which blocks key histone enzymes, could restore DIPG histone function to a more normal state.
Panobinostat is already in early clinical testing.
However, Panobinostat's usefulness may be limited as cancer cells can learn to evade its effects. Monje's team wants to identify other possible drugs - and drug combinations - that could affect these cancers.
"Very few cancers can be treated by a single drug. We've known for a long time that we would need more than one treatment option for DIPG. The challenge is prioritizing the right ones when there are thousands of potential options. We're hopeful that this combination will help these children."
Michelle Monje PhD, Stanford University School of Medicine, Stanford, California, USA and senior author of the study.
NCATS' robotics-enabled high-throughput screening technologies rapidly test thousands of different drugs and drug combinations in a variety of ways. Scientists can examine the most promising single drugs and combinations to determine the most effective doses of each drug and learn more about the possible mechanisms by which these drugs act.
NCATS researchers first studied the effects of single approved drugs and compounds under investigation on DIPG patient cell models grown in the laboratory. They focused on agents that could both kill DIPG cells and cross the protective blood-brain barrier to be effective in patients. The team then tested the most effective single agents in various combinations.
"Such large, complex drug screens take a tremendous collaborative effort," said Thomas, also a senior study author. "NCATS was designed to bring together biologists, chemists, engineers and data scientists in a way that enables these technically challenging studies."
While there were multiple promising drug screen outcomes, the team focused on the combination of histone deacetylase inhibitors (like panobinostat) with drugs called proteasome inhibitors (such as marizomib). Proteasome inhibitors block cells' normal protein recycling processes.
The panobinostat-marizomib combination was highly toxic to DIPG cells in several models including: tumor cell cultures representing the main genetic subtypes of the disease; and, in mice with cells transplanted from patient tumors.
The panobinostat-marizomib combination also reduced tumor size in mice and increased their survival. A similar response was found in spinal cord and thalamic DMG models developed from cells grown in culture from patient cells.
Mechanisms at play
The screening studies also provided important clues to the ways the drugs were working. Building on these data, the collaborative team subsequently conducted a series of experiments that showed the DIPG cells responded to these drugs by turning off a biochemical process in the cell's mitochondria that is partly responsible for creating ATP, which provides energy to cells. The drug combination essentially shuts down tumor cell ATP production.
"The panobinostat-marizomib drug combination exposed an unknown metabolic vulnerability in DIPG cells we didn't expect. It represents an exciting new avenue to explore in developing future treatment strategies for diffuse midline gliomas."
Grant Lin PhD, Stanford University School of Medicine and first author.
Clinical Trials Planned for Drug Combinations & Marizomib
"Many drugs we test have multiple effects on DIPG cells. Panobinostat, for example, inhibits a specific enzyme, but has other mechanisms working in tumor cells that may contribute to its effectiveness. We're still trying to understand the various Achilles heels in these cancer cells. This work is an important step in translating our preclinical data into patients," explains Katherine Warren MD, a senior study author.
Michelle Monje stresses how the panobinostat-marizomib combination might be an important component in a multitherapy strategy, including harnessing the immune system and disrupting factors in the tumor microenvironment that glioma cells depend on to grow. Like Warren, Monje emphasizes the need to better understand how drugs both target and impact DIPG vulnerabilities.
"The idea is to get as many effective tools as possible that have an impact on patients."
Grant Lin PhD
Abstract
Diffuse intrinsic pontine glioma (DIPG) is a lethal childhood brainstem tumour, with a quarter of patients harbouring somatic mutations in ACVR1, encoding the serine/threonine kinase ALK2. Despite being an amenable drug target, little has been done to-date to systematically evaluate the role of ACVR1 in DIPG, nor to screen currently available inhibitors in patient-derived tumour models. Here we show the dependence of DIPG cells on the mutant receptor, and the preclinical efficacy of two distinct chemotypes of ALK2 inhibitor in vitro and in vivo. We demonstrate the pyrazolo [1,5-a] pyrimidine LDN-193189 and the pyridine LDN-214117 to be orally bioavailable and well-tolerated, with good brain penetration. Treatment of immunodeprived mice bearing orthotopic xenografts of H3.3K27M, ACVR1R206H mutant HSJD-DIPG-007 cells with 25 mg/kg LDN-193189 or LDN-214117 for 28 days extended survival compared with vehicle controls. Development of ALK2 inhibitors with improved potency, selectivity and advantageous pharmacokinetic properties may play an important role in therapy for DIPG patients.
Authors
Diana Carvalho, Kathryn R. Taylor, Nagore Gene Olaciregui, Valeria Molinari, Matthew Clarke, Alan Mackay, Ruth Ruddle, Alan Henley, Melanie Valenti, Angela Hayes, Alexis De Haven Brandon, Suzanne A. Eccles, Florence Raynaud, Aicha Boudhar, Michelle Monje, Sergey Popov, Andrew S. Moore, Jaume Mora, Ofelia Cruz, Mara Vinci, Paul E. Brennan, Alex N. Bullock, Angel Montero Carcaboso and Chris Jones.
Acknowledgments
This work was supported by Children with Cancer UK, Abbie’s Army and the DIPG Collaborative, the Lyla Nsouli Foundation and Lucas’ Legacy, the McKenna Claire Foundation and Fondo Alicia Pueyo. The Queensland Children’s Tumour Bank is supported by the Children’s Hospital Foundation. We thank Louise Howell (ICR) for excellent technical assistance. This work was supported by the Xarxa de Bancs de Tumors de Catalunya (XBTC), sponsored by Pla Director d’Oncologia de Catalunya. AMC acknowledges funding from ISCIII-FEDER (CP13/00189). A.B. and A.N.B acknowledge funding from the Amateurs Trust, Roemex Ltd and FOP Friends. The SGC is a registered charity (number 1097737) that receives funds from AbbVie, Bayer Pharma AG, Boehringer Ingelheim, Canada Foundation for Innovation, Eshelman Institute for Innovation, Genome Canada, Innovative Medicines Initiative (EU/EFPIA) [ULTRA-DD grant no. 115766], Janssen, MSD, Merck KGaA, Novartis Pharma AG, Ontario Ministry of Economic Development and Innovation, Pfizer, São Paulo Research Foundation-FAPESP, Takeda and Wellcome [106169/ZZ14/Z]. This study makes use of data generated by Cancer Research UK Genomics Initiative (C13468/A14078). The authors acknowledge NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and the ICR.
Return to top of page.
| |
|
Nov 26 2019 Fetal Timeline Maternal Timeline News
 Glioma cell migration in culture.
|




