|
|
Developmental Biology - Lysosomes
Solving the Mystery of Batten Disease
Batten Disease is the most prevalent cause of brain function loss in children...
Our cells contain numerous sacs full of enzymes that exist only to break down cell waste. These sacs are called lysosomes — and the molecules they break down can be recycled, or discarded through the cell wall into the blood system for removal.
In Batten disease, mutations in the CLN6 gene affects lysosomes, disrupting their ability to eliminate waste materials. Why is still unknown.
So cells begin accumulating waste leading to Batten disease — a lysosomal storage disorder.
Although all cell types can be affected by defects in lysosomal waste management, brain neurons are particularly susceptible.
"Waste accumulation in neurons disturbs many cell processes and eventually results in cell death. This leads to the progressive degeneration of motor, physical and intellectual abilities observed in Batten disease patients."
Lakshya Bajaj PhD, Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, Texas, USA; Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital, Houston, Texas, USA.
Batten disease is one in a family of 13 rare, genetically distinct conditions. Collectively, the most prevalent cause of neurodegenerative disease in children, it affects 1 in 12,500 live births in the USA.
Now, a study led by researchers at Baylor College of Medicine and published in the Journal of Clinical Investigation reveals how the defective CLN6 gene may result in Batten disease.
"People with Batten disease have problems with their cells' ability to clear cellular waste, which then accumulates to toxic levels."
Lakshya Bajaj PhD, workrd on this project while a doctorate student in the laboratory of Dr. Marco Sardiello at Baylor. Today, Bajaj is a post-doctoral associate at Harvard Medical School.
Another Piece of the Batten Disease Puzzle
The connection of CLN6 with Batten disease was a bit of a mystery. This protein is not found in lysosomes, but in the endoplasmic reticulum, a structure inside cells where proteins, including lysosomal enzymes, are made. The endoplasmic reticulum is separate from the lysosomes. So, how do defects in a protein located outside of the lysosomes interfere with lysosomal function?
The Sardiello lab had previously solved a similar mystery involving CLN8, another protein located in the endoplasmic reticulum and whose mutations also cause a type of Batten disease.
"We showed that CNL8 assists the exit of lysosomal enzymes from the endoplasmic reticulum en route to the lysosomes. When CLN8 is defective, the transport of enzymes from their place of synthesis to a final destination is deficient — and lysosomes end up having fewer enzymes to work with."
Marco Sardiello PhD, Associate Professor, Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, Texas, USA; Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital, Houston, Texas, USA; and corresponding author of this work.
CLN6 and CLN8 work together
The clinical manifestations of Batten disease caused by CLN8 mutations and those of Batten disease due to defective CLN6 are remarkably similar. This and other evidence led researchers to suspect CLN6 and CLN8 might be working together.
Their investigations revealed that CLN6 and CLN8 do interact with each other forming a molecular complex that collects lysosomal enzymes at the endoplasmic reticulum and mediates their trafficking towards the lysosomes.
"We propose that CLN8 and CLN6 together herd the enzymes into a hub, a sort of 'bus stop.' Then, CLN8 escorts the enzymes onto the bus en route to the lysosomes, while CLN6 remains at the bus stop. CLN8 returns to the bus stop after delivering the enzymes, and they repeat the process. When CLN6 is defective, the enzymes are not effectively herded into the bus stop and fewer are transported to the lysosomes."
Lakshya Bajaj PhD
The researchers are interested in finding whether other factors are involved in transporting enzymes to lysosomes. Such as whether there are other 'bus conductors or herders' of lysosomal enzymes involved and if defective, may also contribute to Batten disease.
Abstract
Lysosomal enzymes are synthesized in the endoplasmic reticulum (ER) and transferred to the Golgi complex by interaction with the Batten disease protein CLN8 (ceroid lipofuscinosis, neuronal, 8). Here we investigated the relationship of this pathway with CLN6, an ER-associated protein of unknown function that is defective in a different Batten disease subtype. Experiments focused on protein interaction and trafficking identified CLN6 as an obligate component of a CLN6-CLN8 complex (herein referred to as EGRESS: ER-to-Golgi relaying of enzymes of the lysosomal system), which recruits lysosomal enzymes at the ER to promote their Golgi transfer. Mutagenesis experiments showed that the second luminal loop of CLN6 is required for the interaction of CLN6 with the enzymes but dispensable for interaction with CLN8. In vitro and in vivo studies showed that CLN6 deficiency results in inefficient ER export of lysosomal enzymes and diminished levels of the enzymes at the lysosome. Mice lacking both CLN6 and CLN8 did not display aggravated pathology compared with the single deficiencies, indicating that the EGRESS complex works as a functional unit. These results identify CLN6 and the EGRESS complex as key players in lysosome biogenesis and shed light on the molecular etiology of Batten disease caused by defects in CLN6.
Authors
Lakshya Bajaj, Jaiprakash Sharma, Alberto di Ronza, Pengcheng Zhang, Aiden Eblimit, Rituraj Pal, Dany Roman, John R. Collette, Clarissa Booth, Kevin T. Chang, Richard N. Sifers, Sung Y. Jung, Jill M. Weimer, Rui Chen, Randy W. Schekman and Marco Sardiello.
Acknowledgements
The authors thank H. Bellen, H. Zoghbi, M. Wang, and T. Eissa for helpful discussion and K. Venkatachalam and H. Jafar-Nejad for critical reading of the manuscript. This work was supported by NIH grants NS079618 and GM127492 (to MS) and grants from the Gwenyth Gray Foundation, Beyond Batten Disease Foundation, and NCL-Stiftung (to MS). This project was supported in part by IDDRC grant number 1U54 HD083092 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Core: Integrated Microscopy). This project was supported by the Integrated Microscopy Core and the Proteomics Core at Baylor College of Medicine with funding from NIH (DK56338, CA125123), CPRIT (RP150578, RP170719), the Dan L. Duncan Comprehensive Cancer Center, and the John S. Dunn Gulf Coast Consortium for Chemical Genomics.
This work was supported by NIH grants NS079618 and GM127492 and grants from the Gwenyth Gray Foundation, Beyond Batten Disease Foundation and NCL-Stiftung. This project was supported in part by IDDRC grant number 1U54 HD083092 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Integrated Microscopy Core and the Proteomics Core at Baylor College of Medicine with funding from NIH (DK56338, and CA125123), CPRIT (RP150578, RP170719), the Dan L. Duncan Comprehensive Cancer Center and the John S. Dunn Gulf Coast Consortium for Chemical Genomics.
The authors have declare that no conflict of interest exists.
Return to top of page.
|
|
Jul 17 2020 Fetal Timeline Maternal Timeline News
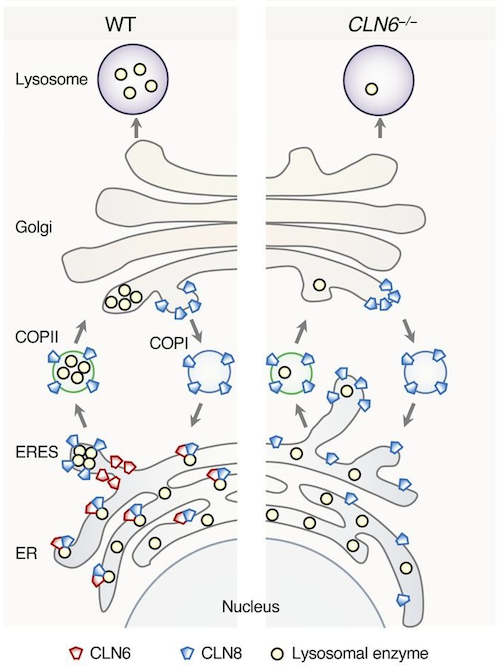 Mice without both CLN6 and CLN8 did not display aggravated pathology compared with single deficiencies, indicating that the EGRESS complex works as a functional unit. These results identify CLN6 and the EGRESS complex as key players in lysosome biogenesis and shed light on the molecular etiology of Batten disease caused by defects in CLN6.
CREDIT The authors.
|
|

