|
|
Developmental Biology - Heart Regeneration
Atlas of Fetal Heart Development
A new study reports a potential therapeutic target that might promote heart cell regeneration even before birth...
Children born with hypoplastic left heart syndrome (HLHS), require a series of major surgical procedures to survive. But even with a repaired heart, as many as one in four can die from complications before age 25. These details were published online Aug. 17, 2020, in the journal Cell Stem Cell.
Mingxia Gu MD PhD has worked for years to find ways to prompt healing in damaged heart tissues. She was formerly at Stanford University, but joined the Center for Stem Cell & Organoid Medicine (CuSTOM) and the Division of Pulmonary Biology at Cincinnati Children's earlier this year.
In this study, a team led by Gu and co-first authors Yifei Miao PhD, and Lei Tian PhD, used advanced single-cell transcriptomic analysis to develop a human heart "atlas" tracking a full chorus of signals endocardial cells send out as the fetal heart develops during pregnancy.
Comparing this atlas of healthy heart development - to data from lab-created induced pluripotent stem cells (featuring HLHS defects) - reveals a collection of abnormalities in endocardial cells that disrupt healthy heart formation.
Endocardial cells play pivotal roles in heart development and disease as they sense blood flow in the heart to interact with surrounding muscle cells, giving rise to key heart structures including: atrioventricular valves and atrial and membranous ventricular septa.
Tracing HLHS-related disruptions to these cells represents a change in thinking compared to efforts focused on myocardial cells, which form the beating muscle of the heart.
Hopeful news is the team found a potential therapeutic target that can be drugged to improve endocardial function, regenerating cardiac valves, septum and coronary vessels, and eventually increasing heart chamber size — which could possibly reduce the need for multiple surgeries after birth.
What Is HLHS?
Hypoplastic left heart syndrome or HLHS, results in infants born with a severely malformed cardiac left ventricle, the heart's main pumping chamber. HLHS accounts for about 3% of all congenital heart defects, with a prevalence rate of 1 to 1.5 cases per 5,000 live births in the United States.
• Treatment requires three surgeries to reroute blood flow so that the right ventricle can support all the work the heart must perform.
• The unusual amount of pressure the repaired heart endures, plus other factors, can lead to heart failure that requires organ transplantation to treat.
For several years, scientists have tried various ways to use a person's own stem cells to help damaged hearts heal. Most early efforts have had little success, especially for adults.
In children, however, heart tissues continue to grow, which makes scientists more hopeful that stem cell therapies, or other treatments, might help improve daily health and life expectancy.
Treatment Target: FN1
Gu and colleagues found the gene fibronectin (FN1) was significantly downregulated in HLHS endocardium. Various genetic abnormalities combined to impede FN1, leading to impaired valve formation and poor cardiomyocyte growth and maturation.
Given that fetal gene editing is not an option for human infants, more research is needed to determine whether a treatment to replace the missing functions of FN1 in HLHS can be developed for use after a child is born.
"Our discoveries provide a new facet to the pathogenesis of HLHS and an alternative angle for early intervention and cardiac regeneration in HLHS. The significance of this finding is that by discovering this gene's role, we now have a target for future study."
Mingxia Gu MD PhD, Department of Pediatrics, Division of Pediatric Cardiology and Vera Moulton Wall Center for Pulmonary Vascular Disease; Stanford Cardiovascular Institute, Stanford School of Medicine, Stanford, California; Perinatal Institute, Division of Pulmonary Biology, Cincinnati Children’s Hospital Medical Center; Center for Stem Cell and Organoid Medicine, CuSTOM, Division of Developmental Biology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Abstract Highlights
• Single-cell RNA-seq identifies an abnormal endocardial population in HLHS
• HLHS endocardial cells show impaired endothelial to mesenchymal transition
• Endocardial defects in HLHS contribute to suppressed myocardial growth and maturation
• Reduction of FN1 underlies endocardial and myocardial dysfunctions in HLHS
Summary
Hypoplastic left heart syndrome (HLHS) is a complex congenital heart disease characterized by abnormalities in the left ventricle, associated valves, and ascending aorta. Studies have shown intrinsic myocardial defects but do not sufficiently explain developmental defects in the endocardial-derived cardiac valve, septum, and vasculature. Here, we identify a developmentally impaired endocardial population in HLHS through single-cell RNA profiling of hiPSC-derived endocardium and human fetal heart tissue with an underdeveloped left ventricle. Intrinsic endocardial defects contribute to abnormal endothelial-to-mesenchymal transition, NOTCH signaling, and extracellular matrix organization, key factors in valve formation. Endocardial abnormalities cause reduced cardiomyocyte proliferation and maturation by disrupting fibronectin-integrin signaling, consistent with recently described de novo HLHS mutations associated with abnormal endocardial gene and fibronectin regulation. Together, these results reveal a critical role for endocardium in HLHS etiology and provide a rationale for considering endocardial function in regenerative strategies.
Authors
Yifei Miao, Lei Tian, Marcy Martin, Sharon L. Paige, Francisco X. Galdos, Jibiao Li, Alyssa Klein, Hao Zhang, Ning Ma, Yuning Wei, Maria Stewart, Soah Lee, Jan-Renier Moonen, Bing Zhang, Paul Grossfeld, Seema Mital, David Chitayat, Joseph C. Wu,Marlene Rabinovitch, Timothy J. Nelson, Shuyi Nie, Sean M. Wu and Mingxia Gu.
Return to top of page.
| |
|
Aug 21 2020 Fetal Timeline Maternal Timeline News
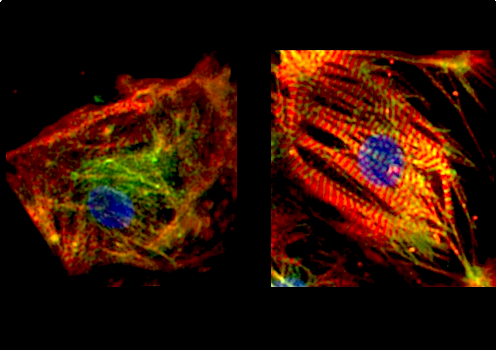 LEFT image of a HLHS-affected fetal mouse heart without FN1 gene. Few bright red muscle bands. RIGHT image of mouse fetal heart with active FN1 gene, showing bright RED cardiac muscle fibers. CREDIT Cincinnati Children's
|



