|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive Mar 23, 2015
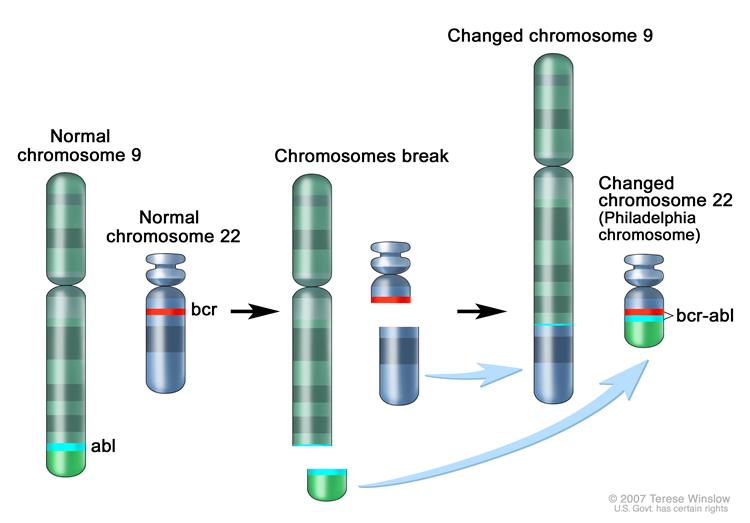
The Philadelphia chromosome is a translocation between the ABL-1 oncogene
(on the long arm of chromosome 9) and the breakpoint cluster region (BCR)
(on the long arm of chromosome 22), resulting in the fusion gene BCR-ABL.
Image Credit: The National Cancer Institute
|
|
|
|
|
|
Infant leukemia results from one rearranged gene
Pediatric Cancer Genome Project reports that a highly aggressive form of leukemia in infants has surprisingly few mutations except for a chromosome rearrangment affecting only one gene.
The finding suggests that targeting this rearrangment is likely key to improved survival. The research appears in the scientific journal Nature Genetics.
The work is the most comprehensive analysis yet of this rare and aggressive subtype of pediatric acute lymphoblastic leukemia (ALL). ALL occurs during the first year of life and sometimes is diagnosed at birth.
Up to 80 percent of the leukemia cells in ALL infants have an MLL gene fused to another gene on a different chromosome. This fused MLL gene goes on to encode an abnormal protein — one that is key in transforming normal blood cells into leukemic cells.
Researchers used whole genome sequencing and other techniques to identify the genetic alterations in 65 infants with ALL, including 47 with the MLL rearrangement. Scientists were surprised to find that despite being an aggressive leukemia, the MLL rearrangment subtype had among the lowest mutation rates reported for any cancer.
"These results show that to improve survival for patients with this aggressive leukemia, we need to develop drugs that target abnormal proteins produced by the MLL fusion gene, or that interact with the abnormal MLL fusion protein, in order to shut down the cell machinery driving tumors. That will not be easy, but this study found no obvious cooperating mutations to target."
James R. Downing MD, St. Jude president and chief executive officer, and senior co-corresponding author.
St. Jude researchers are working to identify compounds and combination therapies to improve cure rates for infants with the MLL rearrangement. Nationally, 85 percent of pediatric ALL patients now enjoy long-term, cancer free survival compared to 28 to 36 percent of infants with the high-risk subtype.
"We frequently associate a cancer's aggressiveness with its mutation rate, but this work indicates that the two don't always go hand-in-hand," said co-author Richard K. Wilson, Ph.D., director of The Genome Institute at Washington University School of Medicine in St. Louis. "Still, our findings provide a new direction for developing more effective treatments for these very young patients."
The other corresponding authors are Tanja Gruber MD, PhD, assistant member in the St. Jude Department of Oncology, and Anna Andersson, Ph.D., formerly of St. Jude and now of Lund University, Sweden. Andersson and Jing Ma, Ph.D., of the St. Jude Department of Pathology, are co-first authors.
Almost half of infants with MLL rearranged ALL had activating mutations in a biochemical pathway called the tyrosine kinase-phosphoinositide-3-kinase (PI3K)-RAS pathway. Surprisingly, the mutations were often present in only some of the leukemic cells. Researchers analyzed leukemia cells in infants whose cancer returned after treatment and found that at the time of relapse the cells lacked the pathway mutations.
"The fact that the mutations were often lost at relapse suggests that patients are unlikely to benefit from therapeutically targeting these mutations at diagnosis,"
James R. Downing MD
Researchers also found that older pediatric leukemia patients with the MLL rearrangement had significantly more mutations than infants.
Almost half of the older children had mutations in genes that encode epigenetic regulatory proteins. Epigenetic proteins influence activation of other genes. "While MLL belongs to a family of genes that encode epigenetic regulatory proteins, there was a striking difference between infants and older children regarding the frequency of mutations in other epigenetic regulatory genes," Andersson said.
"This observation raises the possibility of a fundamental difference in the cell targeted for transformation in infants versus older patients.
"Our working hypothesis is that in infants the MLL rearrangement occurs in a developing blood cell, a prenatal progenitor cell, which requires fewer additional mutations to fully transform into leukemia.
"In contrast, in older patients the MLL rearrangement isn't enough on its own."
Tanja Gruber MD, PhD, assistant member, St. Jude Department of Oncology
Abstract
Infant acute lymphoblastic leukemia (ALL) with MLL rearrangements (MLL-R) represents a distinct leukemia with a poor prognosis. To define its mutational landscape, we performed whole-genome, exome, RNA and targeted DNA sequencing on 65 infants (47 MLL-R and 18 non–MLL-R cases) and 20 older children (MLL-R cases) with leukemia. Our data show that infant MLL-R ALL has one of the lowest frequencies of somatic mutations of any sequenced cancer, with the predominant leukemic clone carrying a mean of 1.3 non-silent mutations. Despite this paucity of mutations, we detected activating mutations in kinase-PI3K-RAS signaling pathway components in 47% of cases. Surprisingly, these mutations were often subclonal and were frequently lost at relapse. In contrast to infant cases, MLL-R leukemia in older children had more somatic mutations (mean of 6.5 mutations/case versus 1.3 mutations/case, P = 7.15 × 10−5) and had frequent mutations (45%) in epigenetic regulators, a category of genes that, with the exception of MLL, was rarely mutated in infant MLL-R ALL.
The other authors are Xiang Chen, Amanda Larson Gedman, Jinjun Dang, Joy Nakitandwe, John Easton, Richard Kriwacki, Michael Rusch, Gang Wu, Yongjin Li, Heather Mulder, Susana Raimondi, Stanley Pounds, Guolian Kang, Lei Shi, Jared Becksfort, Pankaj Gupta, Debbie Payne-Turner, Bhavin Vadodaria, Kristy Boggs, Donald Yergeau, Jayanthi Manne, Guangchun Song, Michael Edmonson, Cheng Cheng, Deqing Pei, Ching-Hon Pui, Sheila Shurtleff, Charles Mullighan and Jinghui Zhang, all of St. Jude; Jianmin Wang, Linda Holmfeldt, Matthew Parker, Robert Huether, Panduka Nagahawatte and Lei Wei, all formerly of St. Jude; Rosemary Sutton and Nicola Venn, both of the University of New South Wales, Sydney, Australia; Albert Chetcuti, Amanda Rush and Daniel Catchpoole, all of the Children's Hospital at Westmead, Sydney, Australia; Jesper Heldrup, of Lund University Hospital, Lund, Sweden; Thoas Fioretos, of Lund University; and Charles Lu, Li Ding and Elaine Mardis, all of Washington University.
The study was funded in part by the Pediatric Cancer Genome Project, including Kay Jewelers, a lead sponsor; a grant (CA021765) from the National Institutes of Health; the Swedish Childhood Cancer Society; the Swedish Research Council; the Swedish Cancer Society; BioCARE; the Gunnar Nilsson Cancer Foundation and ALSAC.
St. Jude Children's Research Hospital
St. Jude Children's Research Hospital is leading the way the world understands, treats and defeats childhood cancer and other life-threatening diseases. It is the only National Cancer Institute-designated Comprehensive Cancer Center devoted solely to children. Treatments developed at St. Jude have helped push the overall childhood cancer survival rate from 20 percent to 80 percent since the hospital opened more than 50 years ago. St. Jude is working to increase the overall survival rate for childhood cancer to 90 percent in the next decade. St. Jude freely shares the breakthroughs it makes, and every child saved at St. Jude means doctors and scientists worldwide can use that knowledge to save thousands more children. Families never receive a bill from St. Jude for treatment, travel, housing and food--because all a family should worry about is helping their child live. To learn more, visit stjude.org or follow St. Jude at @stjuderesearch.
Washington University
Washington University School of Medicine's 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children's hospitals. The School of Medicine is one of the leading medical research, teaching and patient-care institutions in the nation, currently ranked sixth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children's hospitals, the School of Medicine is linked to BJC HealthCare.
Return to top of page
|
