|
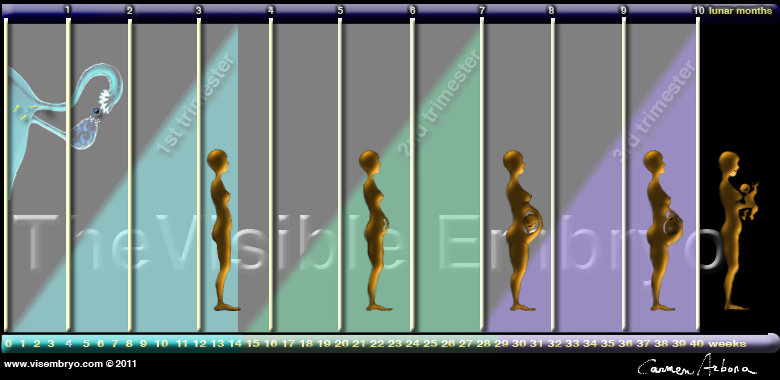
CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
Home | Pregnancy Timeline | News Alerts |News Archive Apr 6, 2015
|
New clues on origin of Hirschsprung's disease The results appear in the April 2 issue of the American Journal of Human Genetics.
The disease arises early in development when nerves that should control the colon fail to grow. These nerves are part of the enteric (a mesh-like system of neurons that governs the function of the gastrointestinal system) nervous system, separate from the central nervous system. The genetic causes of Hirschsprung's disease are complex, making it an interesting case study for researchers like Aravinda Chakravarti, Ph.D., a professor in the Johns Hopkins University School of Medicine's McKusick-Nathans Institute of Genetic Medicine. His research group began studying the condition in 1990. By 2002, his group performed the first-ever genomewide association study to identify common variants linked to the disease. But while Chakravarti's and other groups have identified several gene variants associated with Hirschsprung's, these variants do not explain most cases of the disease. So Chakravarti and his colleagues conducted a genomewide study of genetic markers of more than 650 people with Hirschsprung's disease as compared with their parents and a healthy, unaffected control group. The other finding was of a variant located near genes known for making several semaphorins — proteins that guide developing nerve cells toward their final targets. Through studies in mice and zebrafish, researchers found that semaphorins are active in the developing enteric nervous system. They interact with Ret in a system of pathway signals.
No clinical genetic test yet exists for Hirschsprung's disease. So far, most of the genetic variants connected to Hirschsprung's are relatively common and are associated with less severe forms of the disease. So the hunt will continue for those rare gene variants of the more severe cases. Abstract Other authors on the paper are Qian Jiang, Stacey Arnold, Betty Doan, Ashish Kapoor, Albee Yun Ling, Maria X. Sosa, Moltu Guy, Krishna Praneeth Kilambi, Qingguang Jiang, Grzegorz Burzynski, Kristen West, Seneca Bessling, Jeffrey J. Gray and Andrew S. McCallion of The Johns Hopkins University; Tiffany Heanue and Vassilis Pachnis of the MRC National Institute for Medical Research; Paola Griseri and Isabella Ceccherini of the Istituto Gaslini; Jeanne Amiel and Stanislas Lyonnet of the French National Institute of Health and Medical Research and Paris Descartes University-Sorbonne Paris Cite; Raquel M. Fernandez and Salud Borrego of the University of Seville; Joke B.G.M. Verheij of the University of Groningen; and Robert M.W. Hofstra of the University of Rotterdam. Affymetrix provided arrays used in the study described in this publication. Aravinda Chakravarti was a paid member of the Advisory Board of Affymetrix (2000-2013). This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
|
||||||||||||||||||||||||||||