|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive May 6, 2015
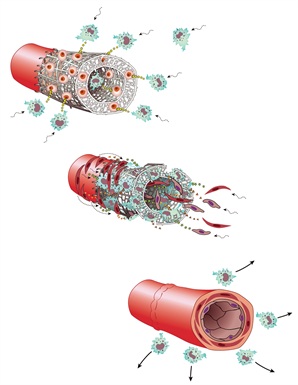
Pediatric surgeons Christopher Breuer and Toshiharu Shinoka used biodegradable tubular scaffolds seeded with a young patient’s bone marrow cells to engineer new blood vessels. The bone marrow cells disappear, but first stimulate an inflammatory response that attracts immune cells to the graft (top). The immune cells then attract epithelial and smooth muscle cells to the dissolving graft (middle) that eventually form the new vessel (bottom).
Image Credit: Yale Medicine |
|
|
|
|
|
Engineering new blood vessels is closer to reality
New research suggests that suppressing parts of the human innate immune system helps engineered vascular grafts become fully functional blood vessels.
Scientists have moved a step closer toward coaxing the body into producing its own replacement blood vessels. This comes after discovering that suppressing parts of our innate immune system can increase the chances of success for tissue engineered vascular grafts.
In a report appearing in the May 2015 issue of The FASEB Journal scientists show they can control the reaction of natural killer cells, platelets and the acute inflammatory response to a graft. They also show they can reduce the abnormal narrowing of the grafts, called stenosis, the cause of most blood vessel failures. This discovery sets the stage for a second, more successful, generation of tissue engineered grafts.
"Our aim is to extend these findings toward the development of a safe and effective tissue engineered vascular grafts for the management of congenital heart disease. We hope that our translational approach is applicable to other areas of regenerative medicine and a model for investigators in the field."
Cameron Best PhD, Tissue Engineering and Surgical Research Department, Research Institute at Nationwide Children's Hospital, Columbus, Ohio.
Best and colleagues made this discovery after observing that immunodeficient mice blunt an acute inflammatory response to implanted tissue engineered vascular grafts — and stenosis, or narrowing of the blood vessel, did not occur. Researchers then treated normal (wild type) mice with either (1) a natural killer cell depleting antibody or (2) anti-platelet drugs, reducing the rate of stenosis in each of these models to about half that observed in normal or untreated, mice.
This result suggests supressing acute inflammation, combined with natural killer cells and platelets, are all critical in engineering vascular grafts.
"When most people think of regenerative medicine, they think of growing new hearts or kidneys," said Gerald Weissmann, M.D., Editor-in-Chief of The FASEB Journal. "What most people don't realize is that just being able to engineer new blood vessels would go a long way toward saving lives and alleviating suffering. This research is significant because it identifies what goes wrong with today's engineered blood vessels, and reveals a solution on what to do to fix this problem."
Abstract
The first clinical trial of tissue-engineered vascular grafts (TEVGs) identified stenosis as the primary cause of graft failure. In this study, we aimed to elucidate the role of the host immune response in the development of stenosis using a murine model of TEVG implantation. We found that the C.B-17 wild-type (WT) mouse (control) undergoes a dramatic stenotic response, which is nearly completely abolished in the immunodeficient SCID/beige (bg) variant. SCID mice, which lack an adaptive immune system due to the absence of T and B lymphocytes, experienced rates of stenosis comparable to WT controls (average luminal diameter, WT: 0.071 ± 0.035 mm, SCID: 0.137 ± 0.032 mm, SCID/bg: 0.804 ± 0.039 mm; P < 0.001). The bg mutation is characterized by NK cell and platelet dysfunction, and systemic treatment of WT mice with either NK cell–neutralizing (anti–NK 1.1 antibody) or antiplatelet (aspirin/Plavix [clopidogrel bisulfate]; Asp/Pla) therapy achieved nearly half the patency observed in the SCID/bg mouse (NK Ab: 0.356 ± 0.151 mm, Asp/Pla: 0.452 ± 0.130 mm). Scaffold implantation elicited a blunted immune response in SCID/bg mice, as demonstrated by macrophage number and mRNA expression of proinflammatory cytokines in TEVG explants. Implicating the initial innate immune response as a critical factor in graft stenosis may provide a strategy for prognosis and therapy of second-generation TEVGs.—Hibino, N., Mejias, D., Pietris, N., Dean, E., Yi, T., Best, C., Shinoka, T., and Breuer, C. The innate immune system contributes to tissue-engineered vascular graft performance.
Details: Narutoshi Hibino, Dane Mejias, Nicholas Pietris, Ethan Dean, Tai Yi, Cameron Best, Toshiharu Shinoka, and Christopher Breuer. The innate immune system contributes to tissue-engineered vascular graft performance. FASEB J. doi:10.1096/fj.14-268334
Receive monthly highlights from The FASEB Journal by e-mail. Sign up at http://www.faseb.org/fjupdate.aspx. The FASEB Journal is published by the Federation of the American Societies for Experimental Biology (FASEB). It is the world's most cited biology journal according to the Institute for Scientific Information and has been recognized by the Special Libraries Association as one of the top 100 most influential biomedical journals of the past century.
FASEB is composed of 27 societies with more than 120,000 members, making it the largest coalition of biomedical research associations in the United States. Our mission is to advance health and welfare by promoting progress and education in biological and biomedical sciences through service to our member societies and collaborative advocacy.
Return to top of page
|
