|
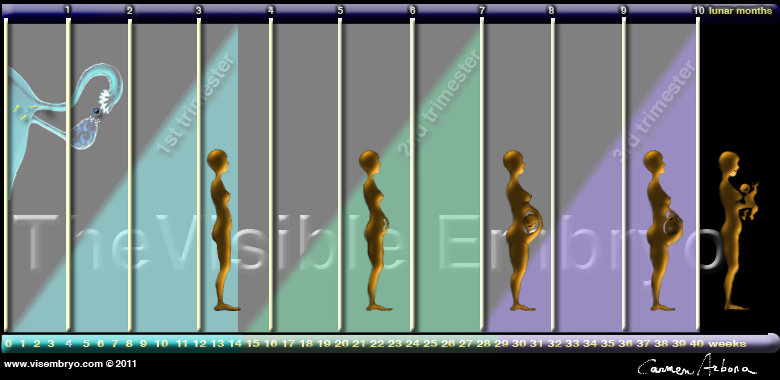
CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
Home | Pregnancy Timeline | News Alerts |News Archive May 12, 2015
|
How our brain plugs in vision stabilization Just as most cameras have an autostabilization feature to compensate for movement during picture taking, our eyes execute an imperceptible reflex that prevents our vision from blurring when we, or our field of vision, are in motion. But before that reflex can work, wirelike projections (axons) of specialized nerve cells must travel from the retina to an exact area of the developing embryonic brain. New research, published in the journal Neuron, describes how these axons make their connection. The finding has implications for treatment of eye movement disorders as well as regeneration of damaged vision-sensing nerve cells.
The neurons in question are a type of direction-sensitive ganglion found in the retina. Each subset of these neurons is in charge of monitoring vertical, horizontal, forward and backward movements. Kolodkin and his group identified the cells that specifically report slow, vertical movement to the brain — what one might experience looking straight ahead while riding a Ferris wheel. Recent work from another research group revealed that slow, vertical movement cells connect their axons to an area of the brain called the medial terminal nucleus (MTN), but not how they reached the MTN during fetal development. For years, Kolodkin has been studying how nerve cell axons arrive at precise locations in the brain. As part of his work, his group discovered guidance proteins called semaphorins (Semas). Their previous work had shown them that the Sema6A protein is required for axon connections within the visual system of the brain. So, the researchers began looking for Sema6A protein in their vertically sensitive cells. After identifying Sema6A was present on the membranes of mouse cells, Lu Sun PhD, first author on the study, genetically deleted Sema6A from mice to assess its exact function. The mice displayed as normally developed during their early embryonic period. However, their axons couldn't recognize when they had reached their target in the brain and retracted. This left the mice unable to appropriately adjust their eyes while viewing vertically moving stripes. If Sema6A was helping guide the axons, the researchers determined that something must exist within the target region of the brain for the cells to recognize in order to establish axon connection. As the Sema6A protein usually interacts with a class of proteins called plexins, the team then looked for plexin proteins in the MTN region of the brain. But something was curious to the research team: "Normally, Sema6A acts as a bait for plexins, not the other way around," noted Kolodkin. To determine how plexins were actually working in this scenario, Kolodkin's team genetically removed plexins from retinal neurons, while maintaining normal levels of Sema6A. If axons needed plexins in order to be guided to their brain target region, their removal would make that connection fail. But they didn't. The plexins in the MTN region were sufficient to coax the Sema6A-laden axons home. This establishes that Sema6A acts as a receptor on the axon cells, recognizing plexins in the target region to allow circuits to be formed, explains Kolodkin. The researchers hope identifying these protein connections will help substantiate what is behind eye movement disorders — as well as nerve regeneration. Specifically, the team's use of moving stripes and infrared light to monitor eye movement can be used to show whether vision can be restored in mice where nerve regeneration is required following eye injury. In the future, they also plan to isolate how these circuits ultimately wire muscles that control eye movement. Abstract Highlights Other authors of the report include Colleen Brady, Hugh Cahill, Timour Al-Khindi and Jeremy Nathans of the Johns Hopkins University School of Medicine; Hiraki Sakuta and Masaharu Noda of the National Institute for Basic Biology in Okazaki, Japan; and Onkar Dhande and Andrew Huberman of the University of California, San Diego. This work was supported by grants from the National Eye Institute (RO1 EY022157-01), a Pew Scholar Award and the Howard Hughes Medical Institute.
|
||||||||||||||||||||||||||||