|
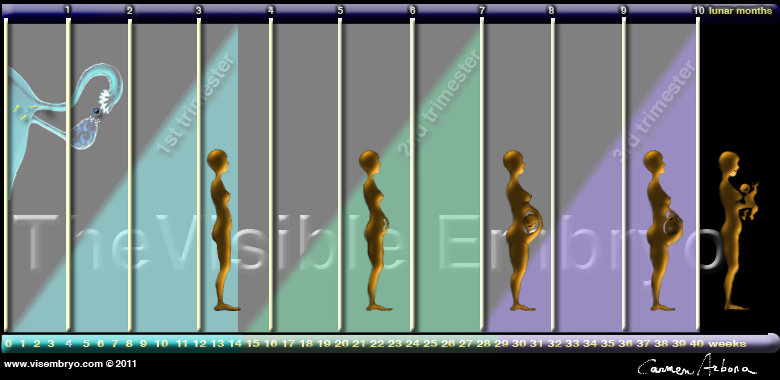
CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
Home | Pregnancy Timeline | News Alerts |News Archive Jun 3, 2015
|
Why and how to barcode thousands of cells When it comes to the tissues in our bodies, which cells do what, is almost always misleading. Scientists know there isn't just one cell type in an organ or any tissue. Scientists run tests to determine what molecules are in their samples. This is useful information, but it doesn't tell where those molecules originally came from. It only provides an average of the type of cells within that sample.
The trouble is, it's expensive, time-consuming and tricky to characterize tissues one cell, or cell type, at a time. Kirschner and Steven McCarroll, assistant professor of genetics at Harvard Medical School (HMS), now report In separate papers — McCarrol in the journal Cell1 and Kirschner also in the journal Cell2 — that each of their labs has developed a system to identify individual cells. Each lab announced a high-throughput technique to quickly and inexpensively assign unique genetic barcodes to every cell in a sample. As a result, scientists can analyze complex tissues by profiling each individual cell without having to average all cells. "Different cells in a tissue use the same genome in amazingly diverse ways: to engineer specialized cell shapes, accomplish diverse feats of physiology, and mount distinct functional responses to the same stimulus. These techniques will finally let science understand how biological systems operate at that single-cell level," said McCarroll, who is also director of genetics for the Stanley Center for Psychiatric Research at the Broad Institute of Harvard and MIT.
The teams expect that each of their techniques will allow biologists to classify cell types and map cell diversity in complex tissues such as those found in the brain. Harvard's Office of Technology Development has been working closely with the researchers to develop patent applications with an eye toward commercializing each method.
But now, Evan Macosko PhD in the McCarroll lab, a Stanley Neuroscience Fellow and HMS instructor in psychiatry at Massachusetts General Hospital, has developed a technique he calls "Drop-seq". Each method uses tiny beads to simultaneously deliver vast numbers of unique DNA barcodes into hundreds of thousands of nanometer-sized water droplets. Thanks to David Weitz's expertise in applied physics and with his assistance, both methods use microfluid devices to encapsulate cells with beads inside of each water droplet. The droplets get created in a tiny assembly line, streaming along a channel the width of a human hair. However, Macosko and Klein make their beads differently. Their droplets get broken up at different steps in their unique processes and each uses diverging aspects of chemistry. But, their results are the same. After running a single batch of cells through "Drop-seq" or "inDrops", scientists can see which genes are expressed in the entire sample — and can sort by each individual cell," says Klein. They can then use computer software to uncover patterns in the overall mix of cells, including which cells have similar gene profiles. This allows scientists to classify which cell types appeared in a sample — and even discover new ones.
Macosko adds: "It finally makes gene expression profiling on a cell-by-cell level, tractable and accessible. I think it's something biologists in a lot of fields will want to use." Rather than competing with each other, the teams believe that having two options available with "Drop-seq" and "inDrops" will benefit the scientific community. "Each method has unique elements that makes it better for different applications. Biologists will be able to choose which one is most appropriate for them," said Macosko. McCarroll, Macosko and colleagues are excited to explore the brain with Drop-seq. This will include the search for new cell types, constructing a global architecture of all brain cell types, and lead to a better understanding of brain development and function as related to disease. Among questions they want to pursue: (1) What are all of the cell types making up the brain? (2) How do these cell types vary in function and response to stimuli? (3) What cell populations are missing or malfunctioning in schizophrenia, autism and other brain disorders?
Meanwhile, Kirschner, Klein and colleagues are keenly interested in stem cell development. "Does a population of cells that we initially think is uniform actually have some substructure?" asks Allon Klein. He's trying to find out through study of immune cells and different types of adult stem cells. "What is the nature of an early developing stem cell? What endows those cells with a pluripotent state? Is gene expression more plastic or does it have a well-defined state that's different from a more mature cell? How is its fate determined?" Using "inDrops", Klein's team has confirmed prior findings that suggest even embryonic stem cells are not uniform. They have found previously undiscovered cell types in embryonic stem cell populations, as well as cells in intermediate stages that may be converting from one cell type to another.
"We have thousands of cells expressing tens of thousands of genes. We can't look in 20,000 directions to pick out interesting features," said Klein. Machine learning is able to do some of the work, and teams already employ new statistical techniques. Still, Kirschner has called on mathematicians and computer scientists to develop new ideas of how to analyze and extract useful information about our biology from this new mountain of data looming on the horizon. McCarrol Team - Drop-seq Cell Kirschner Team - inDrops Cell Allon Klein, Linas Mazutis, Ilke Akartuna, David Weitz and Mark Kirschner have submitted patent applications (US62/065,348, US62/066,188, US62/072,944) for the work described. A patent application has also been filed for the work described by Macosko et al. The Kirschner lab's study was supported by the National Institutes of Health (SCAP Grant R21DK098818), a Career Award at the Scientific Interface from the Burroughs-Wellcome Fund, and a Marie Curie International Outgoing Fellowship (300121). The McCarroll lab's work was supported by the Stanley Center for Psychiatric Research, the Simons Foundation, the National Institutes of Health (P50HG006193, U01MH105960, R25MH094612, F32HD075541), the Klarman Cell Observatory, a Stewart Trust Fellows Award and the Howard Hughes Medical Institute. Microfluidic device fabrication was performed at the Harvard Center for Nanoscale Systems, a member of the National Nanotechnology Infrastructure Network, with support from the National Science Foundation and the Harvard Materials Research Science and Engineering Center.
|
||||||||||||||||||||||||||||