|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive Jul 9, 2015
Eric and Denise Kandal about 1956.
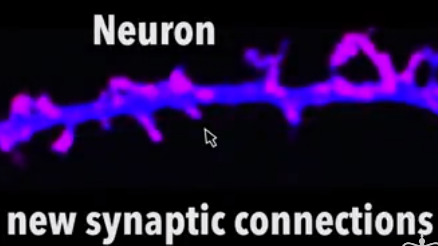
Neuron Images:Laboratory of David Sulzer PhD, Columbia University Medical Center
|
|
|
Long-term memory maintained by prion-like proteins
Research has uncovered evidence of a system in the brain that persistently maintains memories for long periods of time. Paradoxically, it works with the same mechanisms that cause mad cow, kuru, and other brain disorders.
Eric Kandel’s lab at Columbia University Medical Center (CUMC) has uncovered more evidence of how the brain maintains memories for long periods of time. In four papers published in Neuron (1) and Cell Reports (2), Dr. Kandel’s laboratory shows how prion-like proteins – similar to the prions behind mad cow disease in cattle and Creutzfeld-Jakob disease in humans – are critical for maintaining long-term memories in mice, and probably in other mammals.
When long-term memories are created in the brain, new connections are made between neurons to store the memory. But those physical connections must be maintained for a memory to persist, or else they will disintegrate and the memory will disappear within days.
These memory molecules are a normal version of prion proteins, according to research led by Nobel laureate Eric Kandel, MD, University Professor & Kavli Professor of Brain Science, co-director of Columbia’s Mortimer B. Zuckerman Mind Brain Behavior Institute, director of the Kavli Institute for Brain Science, and senior investigator, Howard Hughes Medical Institute, at CUMC.
Prions — derived from the words protein infectious particles — are a unique class of proteins. Unlike other proteins, they not only self-propagate but also induce other proteins to take on their alternative shape. When prions form in a cell, notably a neuron, they cause damage by grouping together in sticky aggregates that disrupt cellular processes.
Prion aggregates are highly stable and accumulate in infected tissue, causing tissue damage and cell death. When the dying cell releases the prion proteins, they are then absorbed by other cells – thus they are considered infectious. These abnormal proteins are known to cause mad cow disease (bovine spongiform encephalopathy). They also are linked to a variety of neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and Huntington’s.
By contrast, functional prion proteins can play a physiological role in the cell and do not contribute to disease.
Kausik Si and Dr. Kandel first identified functional prions in the giant sea slug (Aplysia) and found prions contribute to the maintenance of memory storage. More recently, the Kandel laboratory searched for and found a similar protein in mice, called CPEB3.
In one of many experiments described in the paper by Luana Fioriti (1), the researchers had mice repeatedly navigate a maze in order to create a long-term memory. But when researchers knocked out the animals’ CPEB3 gene two weeks after the memory became permanent, it disappeared.
Memories are stored for the long-term with help from prion-like proteins called CPEB. CPEB prions aggregate and maintain the synapse shape that recorded the memory [“spines” on the lower neuron image]. When CPEB prions are not present or become inactivated, the synapse spines collapse and the memory fades [see top neuron image]. The images were made in the Lab of David Sulzer, PhD, Columbia University Medical Center.
“Like disease-causing prions, functional prions come in two varieties, a soluble form and a form that creates aggregates [CPEB]. When we learn something and form a long-term memory, new synaptic connections are made and the soluble prions in those synapses are converted into aggregated prions. Aggregated prions turn on protein synthesis needed to maintain a memory.
“This ongoing maintenance is crucial. It’s how you remember your first love for the rest of your life.”
As long as these aggregates are present, long-term memories persist. Prion aggregates renew themselves by continually recruiting newly made soluble prions into aggregate groups.
A similar protein exists in humans, suggesting the same mechanism is at work in our brain, but more research is needed. “It’s possible that it has the same role in our memory, but until this is examined, we won’t know,” adds Dr. Kandel. “There are probably other regulatory components involved — long-term memory is a complicated process. So, I doubt this is the only important factor."
Abstract (1) “The Persistence of Hippocampal-based Memory Requires Protein Synthesis Mediated by the Prion-like Protein CPEB3.”
Highlights
•CPEB3 forms aggregates after synaptic activity in the hippocampus
•After aggregation, CPEB3 promotes the translation of AMPA receptors
•CPEB3 conditional knockout mice have impaired long-term memory and LTP
•CPEB3 mediates the persistence of memory through a prion-like mechanism
Summary
Consolidation of long-term memories depends on de novo protein synthesis. Several translational regulators have been identified, and their contribution to the formation of memory has been assessed in the mouse hippocampus. None of them, however, has been implicated in the persistence of memory. Although persistence is a key feature of long-term memory, how this occurs, despite the rapid turnover of its molecular substrates, is poorly understood. Here we find that both memory storage and its underlying synaptic plasticity are mediated by the increase in level and in the aggregation of the prion-like translational regulator CPEB3 (cytoplasmic polyadenylation element-binding protein). Genetic ablation of CPEB3 impairs the maintenance of both hippocampal long-term potentiation and hippocampus-dependent spatial memory. We propose a model whereby persistence of long-term memory results from the assembly of CPEB3 into aggregates. These aggregates serve as functional prions and regulate local protein synthesis necessary for the maintenance of long-term memory.
The complete list of authors is: Eric Kandel, Luana Fioriti, Cory Myers, Yan-You Huang, Xiang Li, Joseph Stephan, Pierre Trifilieff, Stelios Kosmidis, Bettina Drisaldi, and Elias Pavlopoulos (all at CUMC).
This work was supported by grants from the Howard Hughes Medical Institute and the National Institutes of Health (R01 GM070934-06).
Abstract (2) "MicroRNA-22 Gates Long-Term Heterosynaptic Plasticity in Aplysia through Presynaptic Regulation of CPEB and Downstream Targets"
Highlights
•Maintenance of long-term facilitation (LTF) in Aplysia requires upregulation of CPEB
•Serotonin-triggered downregulation of miR-22 permits the upregulation of CPEB in LTF
•Activation of CPEB regulates the translation of target synaptic mRNAs
•Atypical PKC, a CPEB target, synergistically promotes presynaptic LTF maintenance
Summary
The maintenance phase of memory-related long-term facilitation (LTF) of synapses between sensory and motor neurons of the gill-withdrawal reflex of Aplysia depends on a serotonin (5-HT)-triggered presynaptic upregulation of CPEB, a functional prion that regulates local protein synthesis at the synapse. The mechanisms whereby serotonin regulates CPEB levels in presynaptic sensory neurons are not known. Here, we describe a sensory neuron-specific microRNA 22 (miR-22) that has multiple binding sites on the mRNA of CPEB and inhibits it in the basal state. Serotonin triggers MAPK/Erk-dependent downregulation of miR-22, thereby upregulating the expression of CPEB, which in turn regulates, through functional CPE elements, the presynaptic expression of atypical PKC (aPKC), another candidate regulator of memory maintenance. Our findings support a model in which the neurotransmitter-triggered downregulation of miR-22 coordinates the regulation of genes contributing synergistically to the long-term maintenance of memory-related synaptic plasticity.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Three related studies, including more details about CPEB3 function, appeared in June in the online issues Cell Reports:
“SUMOylation is an Inhibitory Constraint that Regulates the Prion-like Aggregation and Activity of CPEB3.” The complete list of authors is: Eric Kandel, Luana Fioriti, Cory Myers, Yan-You Huang, Xiang Li, Joseph Stephan, Pierre Trifilieff, Luca Colnaghi, Stelios Kosmidis, Bettina Drisaldi, and Elias Pavlopoulos (all at CUMC). The study was supported by grants from the Howard Hughes Medical Institute, the Italian Academy for Advanced Studies in America, and the Alexander Bodini Foundation.
“The CPEB3 protein is a functional prion that interacts with the actin cytoskeleton.” The complete list of authors is: Eric Kandel, Joseph S. Stephan, Luana Fioriti, Nayan Lamba, Luca Colnaghi, Kevin Karl, and Irina L. Derkatch (all at CUMC). The study was supported by grants from the Howard Hughes Medical Institute and the National Institutes of Health (R01 GM070934-06).
“MicroRNA-22 gates long-term heterosynaptic plasticity in Aplysia through presynaptic regulation of CPEB and downstream targets.” The complete list of authors is: Eric Kandel, Ferdinando Fiumara (University of Turin, Italy), Priyamvada Rajasethupathy (CUMC), Igor Antonov (CUMC), Stylianos Kosmidis (CUMC), and Wayne Sossin (McGill University, Montreal, Quebec, Canada). The study was supported by a grant from the Howard Hughes Medical Institute.
The authors declare no financial or other conflicts of interest.
Columbia University Medical Center provides international leadership in basic, preclinical, and clinical research; medical and health sciences education; and patient care. The medical center trains future leaders and includes the dedicated work of many physicians, scientists, public health professionals, dentists, and nurses at the College of Physicians and Surgeons, the Mailman School of Public Health, the College of Dental Medicine, the School of Nursing, the biomedical departments of the Graduate School of Arts and Sciences, and allied research centers and institutions. Columbia University Medical Center is home to the largest medical research enterprise in New York City and State and one of the largest faculty medical practices in the Northeast. For more information, visit cumc.columbia.edu or columbiadoctors.org.
Return to top of page
|