|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive Jul 15, 2015
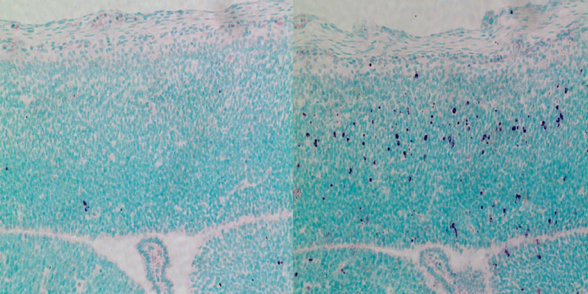
CEP63 depletion increases stem cell death in the developing mouse brain.
RIGHT image shows dying stem cells in purple. Mice are born
with microcephaly, a characteristic feature of Seckel syndrome.
Image Credit: Berta Terré, IRB Barcelona |
|
|
|
|
|
CEP63 Gene key to brain development and fertility
Researchers studying the gene CEP63, found mutated in a rare brain disorder called Seckel syndrome, now know why it causes microcephaly, growth defects and in some cases — male infertility.
Appearing in Nature Communications, scientists at the Institute for Research in Biomedicine — the IRB Barcelona, Spain — identified molecular details about CEP63 that reveal it is involved in brain stem cell division, a key function of brain development. Furthermore, CEP63 is associated with sperm production, unknown until this research.
Rescuing microcephaly in mice
There are no treatment options for microcephaly to date. This defect in brain growth is present in several neurodevelopmental diseases in addition to Seckel Syndrome. "There are diagnostic tests for some of these kinds of pathologies that can be performed during pregnancy. But other than early detection, expectant parents are limited to two choices, either to abort or to continue with the pregnancy, being fully aware of potential outcome," explains Travis Stracker PhD, head of the Genomic Instability and Cancer Lab at IRB Barcelona. "Our research paves the way to explore therapeutic approaches for microcephaly involving the inhibition of the protein p53."
The CEP63 protein triggers death of brain stem cells exhibiting delayed cell division due to their lack of CEP63. These cells enter programmed cell death via the gene p53.
"Cell death due to mutations in CEP63 is the main cause of brain defects. When we prevent cell death by removing p53 from developing [mouse] embryos, the brain develops to its normal size."
Jens Lüders PhD, head of the Microtubule Organization Lab, Institute for Research in Biomedicine (IRB Barcelona), Barcelona, Spain.
This observation paves the way to examine whether inhibiting p53 could provide a future treatment preventing microcephaly in carriers of the CEP63 gene.
"It is too early to say we have a treatment proposal for humans because we are in the first stage of discovery. Also, a normal sized brain does not imply a functional brain. Our next goal is to test the p53 inhibitors currently available on the same mouse models and to characterise and analyse any long-term effects. Furthermore, inhibiting p53 could be harmful as this gene has many functions in normal embryonic development," the researchers warn.
Infertility
The study also revealed that CEP63 is related to fertility in male mice. Researchers discovered the protein is involved in sperm production and, when absent, mice have severe infertility.
"We know that CEP63 depletion leads to problems during meiosis, a specialized type of cell division required for male germ cells to produce sperm."
Travis Stracker PhD, Head of the Institute for Research in Biomedicine — IRB Barcelona, Spain.
"It is an interesting finding because in many cases, infertility is not explainable. This study provides a new molecular perspective to examine," adds Lüders.
Abstract
CEP63 is a centrosomal protein that facilitates centriole duplication and is regulated by the DNA damage response. Mutations in CEP63 cause Seckel syndrome, a human disease characterized by microcephaly and dwarfism. Here we demonstrate that Cep63-deficient mice recapitulate Seckel syndrome pathology. The attrition of neural progenitor cells involves p53-dependent cell death, and brain size is rescued by the deletion of p53. Cell death is not the result of an aberrant DNA damage response but is triggered by centrosome-based mitotic errors. In addition, Cep63 loss severely impairs meiotic recombination, leading to profound male infertility. Cep63-deficient spermatocytes display numerical and structural centrosome aberrations, chromosome entanglements and defective telomere clustering, suggesting that a reduction in centrosome-mediated chromosome movements underlies recombination failure. Our results provide novel insight into the molecular pathology of microcephaly and establish a role for the centrosome in meiotic recombination.
Authors: Marko Marjanovi, Carlos Sánchez-Huertas, Berta Terré, Rocío Gómez, Jan Frederik Scheel, Sarai Pacheco, Philip A. Knobel, Ana Martínez-Marchal, Suvi Aivio, Lluis Palenzuela, Uwe Wolfrum, Peter J. McKinnon, José A. Suja, Ignasi Roig, Vincenzo Costanzo, Jens Lüders, and Travis H. Stracker
(July 2015): DOI: 10.1038/ncomms8676
The study has been financed by the "Plan Nacional" of the Ministry of Economy and Competitiveness and by the European Union programme Marie Curie Actions, through a postdoctoral grant awarded to Marko Marjanovi, first author of the study.
About IRB Barcelona
Founded in 2005 by the Government of Catalonia and the University of Barcelona, the Institute for Research in Biomedicine (IRB Barcelona) is "Severo Ochoa Centre of Excellence", since 2011. The 23 groups and seven scientific platforms are devoted to basic and applied research with the common goal of conducting multidisciplinary projects that address important biomedical problems affecting our society, with special emphasis on cancer, metastasis, Alzheimer, diabetes and rare diseases. The institute is home to more than 400 employees from 36 countries. IRB Barcelona's ultimate objective is to translate research results to the clinic and has already established three biotechnology spin-off companies to this end. This year the IRB Barcelona celebrates its tenth anniversary.
http://www.irbbarcelona.org /@IRBBarcelona / http://www.facebook.com/irbbarcelona
Return to top of page
|
