|
|||||||||||||||
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|
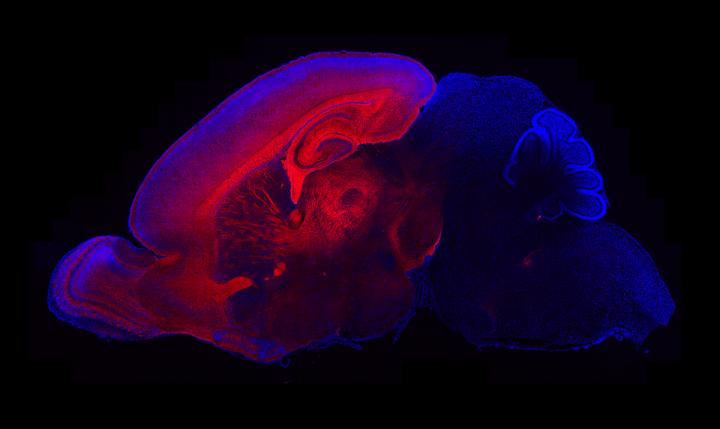
More than half a century after autism was identified by psychiatrist Leo Kanner in 1943, the exact causes of this brain disorder still remain unclear. Now Scripps research has uncovered how mutations in the gene PTEN, mutated in 20% of autism cases, alters early brain development in mice and contributes to macrocephaly or enlarged head. The study is published in the July 15 issue of The Journal of Neuroscience, and focuses on the gene PTEN (Phosphatase and tensin homolog), which is mutated in around 20 percent of individuals with autism spectrum disorder with macrocephaly (enlarged head). Scripps Florida biologist Damon Page and team, observed that mutations in the PTEN gene approximately reflects the percentage of a subgroup of individuals with autism spectrum disorder with enlarged heads. These PTEN mutations lead to increases in two key cell types making up the brain — neurons and glial cells. In adult mice, the number of neurons in the brains of PTEN mutants is virtually the same as in normal mice. However, glia cells — which support neurons — are overrepresented.
Brain overgrowth is a dynamic process. The greatest increase in size occurrs at birth and then again in adulthood. The least overgrowth is in the early juvenile. The team observed that this abnormal growth pattern appears to be sparked by amplification of neuron development. Before birth, neurons are trimmed by a process of programmed cell death (called apoptosis), and glia cells development follows after neurons. "Apoptosis is a natural phenomenon that removes unnecessary neurons during normal brain development," says Research Associate Youjun Chen, the first author of the study and a member of the Page laboratory. "We find it very striking that in brains of PTEN mutant mice, the presence of excess neurons is corrected by excessive apoptosis. After that, excess glia are then made. In adulthood, the number of glial cells continues to increase by more than 20 percent in our [mouse] models." The scientists traced these effects back to an increase in signals from a molecule known as β-Catenin (beta Catenin).
Interestingly, Page noted that in spite of the profound effects of PTEN mutations on brain growth, the mice are largely able to adapt at the level of behavior, with the important exception of social behavior and a few other behaviors relevant to autism spectrum disorder. Page: "Our findings across studies indicate that it may be a multiple-hit process. While abnormal growth puts stress on the developing brain, the brain works hard to compensate. How well an individual can adapt to an abnormal pattern of brain growth may shape their outcome in terms of behavior and cognition. The capacity to adapt may, in turn, be influenced by genetic or environmental factors." Abstract Significance Statement In addition to Page and Chen, other authors of the study, "Pten Mutations Alter Brain Growth Trajectory and Allocation of Cell Types through Elevated β-Catenin Signaling," are Wen-Chin Huang, Julien Sejourne and Amy E. Clipperton-Allen of TSRI. This work was supported by the state of Florida, Ms. Nancy Lurie Marks, the Simons Foundation (grant award 360712), an O'Keeffe Neuroscience Scholars Award and the Fraternal Order of Eagles.
|
||||||||||||||||||||||||||||