|
|
Fetal Timeline Maternal Timeline News News Archive Aug 28, 2015
Zebra fish
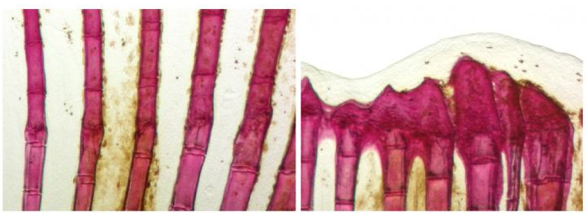
Zebra Fish tail fin
On the left is normal regeneration of fin rays. On the right, irrregular bone formation
in fins with increased production of the signaling protein Sonic Hedgehog.
Image Credit: Developmental Biology, University of Bayreuth.
|
|
| |
|
|
|
How zebrafish rebuild their amputated fins
Fish have the fascinating ability to fully regenerate amputated organs. Unlike us poor humans. Therefore, Zebrafish are a favorite model for studying the processes behind organ regeneration and potential human application.
The zebrafish (Danio rerio) is a popular ornamental fish. When parts of its tailfin are injured by predators, or amputated in a lab, the loss is replaced within three weeks.
Zebrafish fins consist of skin stabilized over a skeleton of bony fin rays, similar to an umbrella supported by metallic stretchers. Fin rays are formed by bone-producing cells called osteoblasts. In order to rebuild an amputated fin, a large number of new osteoblasts are formed by cell divisions of existing osteoblasts. |
|
|
But before this can happen, osteoblasts in the vicinty of the wound must abandon their production of bone material and revert (dedifferentiate) back to a "rejuvenated" earlier stage of development. Specialized mature cells, now become osteoblast precursor cells — a requirement to begin several new cell divisions.
Up to now, little was known about how changes in osteoblasts were brought about, or how zebrafish manage to regenerate the exact shape of a lost fin. Gerrit Begemann PhD, professor at the Developmental Biology unit, University of Bayreuth (Germany), and PhD student Nicola Blum now report progress on both fronts in two articles published in the journal Development.
Their results are likely to excite efforts to regenerate bone tissue and injured organs in humans.
Retinoic acid stimulates the addition of bone material in growing fish — but first, mature osteoblasts have to revert back to an immature osteoblast precursor state, in order to trigger the "on" gene switch for bone making — a reversion that requires retinoic acid levels to drop below a critical level. However, upon fin amputation the tissue beneath the wound initiates a massive amount of retinoic acid to initiate chemical compounds mobilizing cell division in the fin stump. Now, how do mature osteoblasts circumvent this dilemma of too high a retinoic acid level?
The answer was provided by Nicola Blum in the Begermann laboratory. Osteoblasts that participate in regeneration temporaily produce Cyp26b1, an enzyme that destroys and inactivates retinoic acid. With dropping retinoic acid levels provided by Cyp26b1, osteoblasts rewind and turn into precursor cells that contribute to a pool of undifferentiated cells called the blastema. It is these cells - the blastema - that pass through another series of cell divisions, then mature and produce new fin rays. Other parts of the blastema continue to produce retinoic acid.
"This is an elegant mechanism to ensure a gradient of cells experiencing high and low levels of retinoic acid; thereby allowing two processes to run in parallel: (1) Proliferation for the production of all cells to replace the lost structure and (2) osteoblast re-differentiation where the skeleton re-emerges."
Gerrit Begemann PhD, Professor, Developmental Biology, University of Bayreuth (Germany)
Now, how does the exact shape of the fin skeleton regenerate? In order to form new fin rays, the newly formed osteoblasts must align precisely at the extension of existing fin rays in the stump. In their second study, also published in Development, Nicola Blum and Gerrit Begemann broke down the events required for skeletal pattern regeneration.
Osteoblasts are ultimately guided to their targets by a signaling protein called Sonic Hedgehog. Sonic Hedgehog (Shh) is produced locally in the epidermis, a skin-like layer that covers the fin and blastema cells. But Shh signals also only operate in areas free of retinoic acid (as do osteoblast cells), so fin epidermal cells produce Cyp26a1 an enzyme very similar to Cyp26b1.
However, Nicola Blum manipulated retinoic acid levels, affecting Sonic Hedgehog expression at the regenerating site. Thus she revealed that Shh acts as a beacon to osteoblast precursor cells as the consequences of elevated Shh levels were dramatic. Instead of aligning just with existing fin rays, the osteoblast cells also invaded spaces between them, where normally elastic skin forms. Eventually, bone formed at inappropriate positions, sabotaging the regeneration of the original skeletal pattern.
It became obvious that osteoblasts themselves exert a piloting function for other cell types. Mesenchymal cells and blood vessels also have to be directed to appropriate destinations during the fin rebuilding process. If osteoblast precursors are misguided, the cells worsen the rebuild of a functional fin.
"The re-emergence of the skeletal pattern relies on a navigation system with interacting parts. Initially, retinoic acid is inactivated where new rays are to form. This allows the local production of a signal that pilots immature osteoblasts to areas where existing fin rays are to be extended. Interestingly, over the course of regeneration other cell types in the blastema are informed by osteoblast precursor cells to respect the boundaries between emerging fin rays."
Gerrit Begemann PhD
Abstract: Osteoblast de- and redifferentiation is controlled by a dynamic response to retinoic acid during zebrafish fin regeneration
Zebrafish restore amputated fins by forming tissue-specific blastema cells that coordinately regenerate the lost structures. Fin amputation triggers the synthesis of several diffusible signaling factors that are required for regeneration, raising the question of how cell lineage specific programs are protected from regenerative crosstalk between neighboring fin tissues. During fin regeneration, osteoblasts revert from a non-cycling, mature state to a cycling, preosteoblastic state to establish a pool of progenitors within the blastema. After several rounds of proliferation, preosteoblasts redifferentiate to produce new bone. Blastema formation and proliferation are driven by the continued synthesis of retinoic acid (RA). Here we find that osteoblast dedifferentiation and redifferentiation are inhibited by RA signaling and uncover how the bone regenerative program is achieved against a background of massive RA synthesis. Stump osteoblasts manage to contribute to the blastema by upregulating expression of the RA degrading enzyme cyp26b1. Redifferentiation is controlled by a presumptive gradient of RA, in which high RA-levels towards the distal tip of the blastema suppress redifferentiation. We show that this might be achieved through a mechanism involving repression of Bmp signaling and promotion of Wnt/β-catenin signaling. In turn, cyp26b1-positive fibroblast-derived blastema cells in the more proximal regenerate serve as a sink to reduce RA levels, thereby allowing differentiation of neighboring preosteoblasts. Our findings reveal a mechanism explaining how the osteoblast regenerative program is protected from adverse crosstalk with neighboring fibroblasts that advances our understanding of the regulation of bone repair by RA.
Received November 23, 2014.
Accepted July 27, 2015.
Abstract: Retinoic acid signaling spatially restricts osteoblasts and controls ray-interray organization during zebrafish fin regeneration
The zebrafish caudal fin consists of repeated units of bony rays separated by soft interray tissue, an organization that must be faithfully reestablished during fin regeneration. How and why regenerating rays respect ray-interray boundaries, thus extending only the existing bone, has remained unresolved. Here, we demonstrate that a retinoic acid (RA)-degrading niche is established by Cyp26a1 in the proximal basal epidermal layer that orchestrates ray-interray organization by spatially restricting osteoblasts. Disruption of this niche causes preosteoblasts to ignore ray-interray boundaries and to invade neighboring interrays where they form ectopic bone. Concomitantly, non-osteoblastic blastema cells and regenerating blood vessels spread into the interrays, resulting in overall disruption of ray-interray organization and irreversible inhibition of fin regeneration. The cyp26a1-expressing niche plays another important role during subsequent regenerative outgrowth, where it facilitates the Shha-promoted proliferation of osteoblasts. Finally, we show that the previously observed distal shift of ray bifurcations in regenerating fins upon RA treatment, or amputation close to the bifurcation, can be explained by inappropriate preosteoblast alignment and does not necessarily require putative changes in proximodistal information. Our findings uncover a mechanism regulating preosteoblast alignment and maintenance of ray-interray boundaries during fin regeneration.
Received November 23, 2014.
Accepted July 22, 2015.
Return to top of page
|




