|
|||||||||||||||
|
CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Fetal Timeline Maternal Timeline News News Archive Sep 11, 2015
|
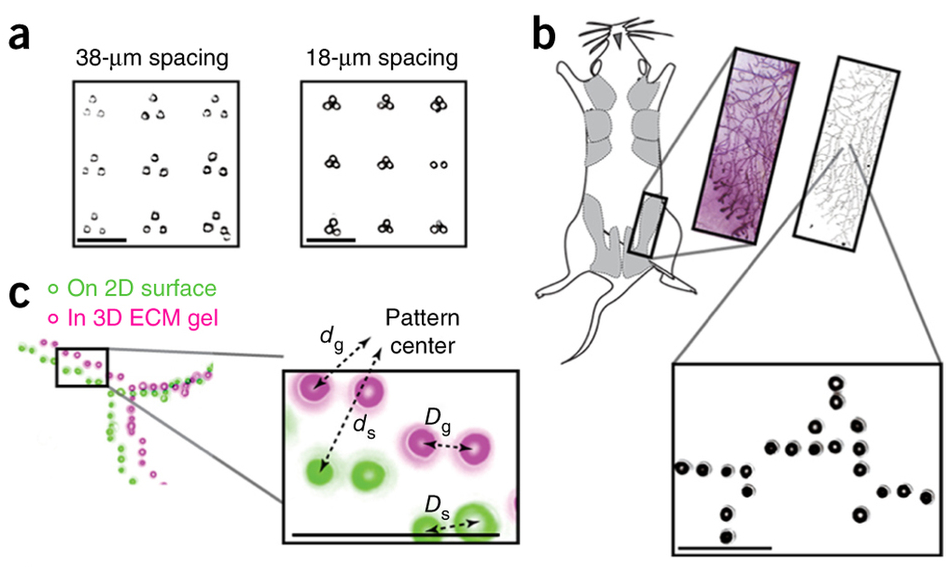
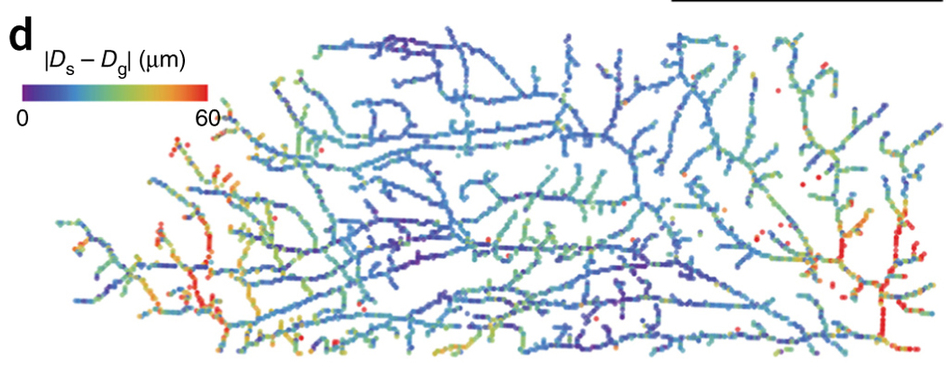
3-D printed organoids - first step to building organs A University of California San Francisco (UCSF) led team has created organoids in a dish to study how particular tissue structures reflect normal growth — or go awry in cancer. Organoids can also be used for therapeutic drug screening and to help teach researchers how to eventually grow whole human organs. The new technique — called DNA Programmed Assembly of Cells (DPAC) is reported in the journal Nature Methods, August 31, 2015. It allows researchers to create arrays of thousands of custom-designed organoids, such as models of human mammary glands, which contain several hundred cells each yet can be built in a matter of hours. There are few limits to the tissues this technology can mimic, explains Zev Gartner PhD, senior author and an Associate Professor of Pharmaceutical Chemistry at UCSF.
Our bodies are made of more than 10 trillion cells of hundreds of different kinds, each playing a unique role in keeping us alive and healthy. The way these cells organize themselves structurally in different organ systems helps coordinate amazingly diverse behaviors and functions that keep our whole biological machine running smoothly. But in diseases such as breast cancer, the breakdown of this order leads to the rapid growth and spread of tumors. Gartner: "Cells aren't lonely little automatons. They communicate through networks to make group decisions. As in any complex organization, you really need to get the group's structure right to be successful, as many failed corporations have discovered. In the context of human tissues, when organization fails, it sets the stage for cancer." Studying how the cells of the mammary gland self-organize, and then break down with disease, has been a challenge. A living organism is often too complex to reveal a specific cause for a particular cell outcome. On the other hand, cells in a dish also lack the critical element of 3-D structure.
To specify the 3-D structure of their organoids, Gartner's team made use of a familiar molecule: DNA. Researchers incubated cells from tiny snippets of single-stranded DNA engineered to slip into a cells' outer membrane. The scientists cover a single cell with these snipets of DNA, much like the fibrous hairs covering a tennis ball. The DNA strands act as a sort of molecular Velcro, in addition to being a bar code that specifies where each cell goes within the organoid. When two separate cells, covered with complementary DNA come into contact, they stick fast to one another. If the DNA sequences don't match, the cells float on by each other. Cells can be incubated with several sets of DNA bar codes to attract multiple partners. To turn these cellular LEGOs into arrays of organoids that can be used for research, Gartner's team lays down the cells in layers, with multiple sets of cells designed to stick to particular partners. Not only does this process allow cells to build up complex tissue components like the mammary gland, but also lends itself to experiments. By being able to specifically add in a single cell with a known cancer mutation to different parts of the organoid, researchers can observe that cells' effect.
In one experiment, the researchers created several groups of mammary epithelial cells to watch for an affect from low levels of the cancer gene RasG12V. They found normal cells grow faster in an organoid with low levels of RasG12V, but required more than one mutant cell to kick-start the abnormally fast growth. They also observed how cells with low expression of RasG12V, when placed at the tip of a tube filled with normal cells, branched and grew and pulled normal cells behind them — just as buds grow from the tip of a tree branch. Gartner plans to use the DPAC organoid technique to investigate mammary gland cellular structure breakdown in tumor metastasis. He also hopes to learn from building simple models of various tissue types, how to ultimately build functional organs and neural circuits.
Abstract Other researchers on the paper include postdoctoral fellow Alex Hughes, PhD, staff researcher Maxwell Coyle, former graduate students Alec Cerchiari, PhD, and Justin Farlow, PhD, and Tejal Desai, PhD, professor of bioengineering, all of UCSF; James Garbe, PhD, a staff scientist at UCSF and the Lawrence Berkeley National Laboratory; and Mark LaBarge PhD, a staff scientist at the Lawrence Berkeley National Laboratory. Funders of the work include the Department of Defense Breast Cancer Research Program, the National Institutes of Health, the Sidney Kimmel Foundation, and the UCSF Program in Breakthrough Biomedical Research. UC San Francisco (UCSF) is a leading university dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. It includes top-ranked graduate schools of dentistry, medicine, nursing and pharmacy, a graduate division with nationally renowned programs in basic, biomedical, translational and population sciences, as well as a preeminent biomedical research enterprise and two top-ranked hospitals, UCSF Medical Center and UCSF Benioff Children's Hospital San Francisco.
|
||||||||||||||||||||||||||||