|
|
Fetal Timeline Maternal Timeline News News Archive Sep 15, 2015
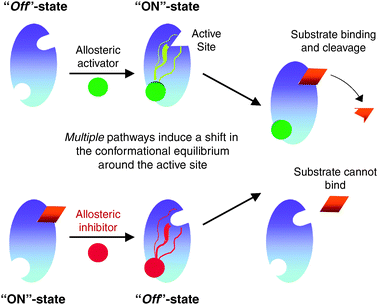
A simplified view of allostery in proteases (enzymes that cut the chemical bond between
two amino acid molecules). (Top) Binding of an activator to an allosteric site creates
a shift in molecular equilibrium - it can be used to predict and explain product(s)
selectivity, mechanisms, and rates of chemical reactions.
Image Credit: Molecular BioSystems Issue 8, 2010
|
|
| |
|
|
|
New molecule found to prevent preterm birth
A molecule named 101.10, inhibits inflammation-induced uterine contractions.
Pre Term births (PTB) are linked to uterine tissue inflammation, which leads to contractions and early labor.
In their search for a means to prevent preterm labor and its complications in deliveries before 37 weeks gestation, researchers at Centre Hospitalier Universitaire Sainte-Justine (CHU Sainte-Justine) and University of Montreal, have discovered an agent that inhibits inflammation.
This discovery is a giant step towards preventing prematurity, the world's leading cause of infant death. Premature birth has potentially severe physical, intellectual and psychological impairment for the 10% of infants born early
The scientists targeted therapeutic agents known to trigger and amplify inflammation — the Interleukin1 group of 11cytokines. In preclinical trials testing therapeutic agents - their testing agents had negligible affect on the contraction of uterine cells, while causing serious side effects to the mother and fetus.
"Interleukin-1's do more than amplify inflammation. Their role is critical in protecting a vulnerable fetus against infection, ensuring cells survive inflammation. Orthosteric antagonists currently available on the market are large molecules that block most of the signaling pathways of Interleukin 1, including some protective mechanisms such as immune system surveillance and cytoprotection, both critical to the fetus."
Mathieu Nadeau-Vallée Ph D candidate in pharmacology, first author of the paper.
Looking for a way to get past this problem, the scientists developed another therapeutic agent which proved much more effective, in addition to being safer. They used an allosteric control which means, regulating a protein by binding a molecule to any site other than the active site of a protein.
"The allosteric modulator we developed work differently. Our molecule is very small. The small size of this molecule allows it to act more selectively on Interleukin-1ß. It specifically blocks the pathway that controls inflammation without interfering with those molecules which exert a protective effect on the immune system."
Sylvain Chemtob MD PhD, neonatologist, investigator and lead author of the study, Professor of Chemistry, University of Montreal.
The article was published online August 24, 2015 in the Journal of Immunology. Dr. Chemtob developed the molecule in collaboration with research associates Christiane Quiniou PhD and Dr William Lubell, Professor of Chemistry at University, Montreal, Canada.
Molecule 101.10, as the scientists have named it, now needs to be tested in humans. For the time being, women with a history of prematurity are candidates for future treatment as evidence shows they are at increased risk for preterm labor.
The research team is looking forward to tests becoming available in the near future that will predict the preterm delivery risk for all women, regardless of their history.
Abstract
Preterm birth (PTB) is firmly linked to inflammation regardless of the presence of infection. Proinflammatory cytokines, including IL-1β, are produced in gestational tissues and can locally upregulate uterine activation proteins. Premature activation of the uterus by inflammation may lead to PTB, and IL-1 has been identified as a key inducer of this condition. However, all currently available IL-1 inhibitors are large molecules that exhibit competitive antagonism properties by inhibiting all IL-1R signaling, including transcription factor NF-κB, which conveys important physiological roles. We hereby demonstrate the efficacy of a small noncompetitive (all-d peptide) IL-1R–biased ligand, termed rytvela (labeled 101.10) in delaying IL-1β–, TLR2-, and TLR4-induced PTB in mice. The 101.10 acts without significant inhibition of NF-κB, and instead selectively inhibits IL-1R downstream stress-associated protein kinases/transcription factor c-jun and Rho GTPase/Rho-associated coiled-coil–containing protein kinase signaling pathways. The 101.10 is effective at decreasing proinflammatory and/or prolabor genes in myometrium tissue and circulating leukocytes in all PTB models independently of NF-κB, undermining NF-κB role in preterm labor. In this work, biased signaling modulation of IL-1R by 101.10 uncovers a novel strategy to prevent PTB without inhibiting NF-κB.
The study was supported by the Global Alliance for the Prevention of Prematurity and Stillbirth (an initiative of the Seattle Children's Hospital) and the Canadian Institutes for Health Research.
About the authors
Mathieu Nadeau-Vallée is a Ph.D. candidate in pharmacology at CHU Sainte-Justine and University of Montreal, who is under the supervision of Dr. Sylvain Chemtob. Dr. Chemtob is a neonatalogist at CHU Sainte-Justine, an investigator in the Fetomaternal and Neonatal Pathologies research axis at CHU Sainte-Justine Research Center and full professor in the Departments of Pediatrics, Ophthalmology and Pharmacology at University of Montreal.
Mathieu Nadeau-Vallée is supported by a scholarship of the Suzanne Véronneau-Troutman Funds associated with the Department of Ophtalmology of University of Montreal and by the Vision Research Network. Dr. Chemtob holds a Canada Research Chair (Vision Science) and the Leopoldine A. Wolfe Chair in translational research in age-related macular degeneration. The University of Montreal is known officially as Université de Montréal.
This work was supported by the Global Alliance for the Prevention of Prematurity and Stillbirth (an initiative of Seattle Children's) and the Canadian Institute of Health Research. M.N.-V. was supported by a scholarship from the Suzanne Veronneau-Troutman Funds associated with the Department of Ophthalmology of University of Montreal and by the Vision Research Network; J.P. was sponsored by the National Council for Scientific and Technological Development under an agreement with the Canadian Bureau for International Education; A.M. was recipient of the Canadian Institute of Health Research Drug Design Training Program and Systems Biology Training Program; F.D. was recipient of the Canadian Institute of Health Research Frederick Banting and Charles Best Canada Graduate Scholarships (Ph.D.), the Fonds de la Recherche en Santé du Québec Ph.D. Scholarship for Health Professional, the Stars Foundation, the CHU Sainte-Justine's Foundation (Ph.D.), and the Hydro-Québec Scholarship for Excellence (Ph.D.); and S.C. holds a Canada Research Chair (Vision Science) and the Leopoldine Wolfe Chair in translational research in age-related macular degeneration.
Return to top of page
|



