|
|||||||||||||||
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
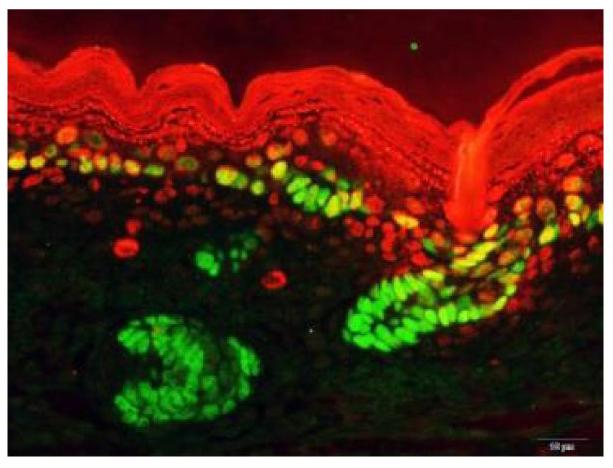
Two genes play major role in growth and cancers Knocking out one or both of these two crucial regulatory genes caused cleft lip, skin barrier defects, and a host of other problems in developing mice, according to research from the Perelman School of Medicine at the University of Pennsylvania. This outcome hints that abnormalities in molecular pathways underlie many birth defects.
Published this week in eLife, the work explains how two genes, Esrp1 and Esrp2, not only play a role in development - but also in cancers. Carstens and his colleagues in 2009 first identified Esrp1 and 2 as molecular switches for alternative splicing — a process in which RNA is transcribed from a gene's DNA. The DNA is cut up and put back together in different arrangements or "alternative splicings." Most mammalian genes use it to enable one gene to produce many protein variations — called isoforms. Which isoform is produced depends on the cell type and other physiologic circumstances. Esrp1 and Esrp2 are specific to epithelial cells which form skin, the inner lining of our gut and lungs, and some other tissues in the body. Carstens and his team since 2009 have shown that Esrp1 and 2 proteins can splice hundreds of different isoforms from epithelial-specific genes. In 2013, they discovered that suppressing Esrp activity enables epithelial cells to detach and develop into new tissue — a process called epithelial-mesenchymal transition (EMT). Obviously EMT is crucial for creating new tissue during embry development and later for healing wounds. Early studies of the two proteins were made in laboratory-cultured cells. In this eLife study, Carstens and his colleagues have engineered mice without Esrp1 or Esrp2 or both. They found that mice born alive but without Esrp1, had cleft palate and cleft lip. Their deformities prevented feeding and all pups died within hours.
Mice lacking only Esrp2 — which likely evolved as a backup copy to cover some functions of Esrp1 — appeared normal if Esrp1 was still present as a gene. Mice lacking both genes had more abnormalities. Their embryos had more profound defects than in the mice missing Esrp1, including craniofacial and forelimb defects, and the complete absence of lungs and salivary glands — two organs largely from epithelial cells. Collaborating with Yi Xing from UCLA, the team catalogued and analyzed how gene patterns in skin cells and how they differed among mice with various Esrp gene knockout combinations, finding hundreds of significant changes. Their analysis suggested that the lack of both Esrp1 and Esrp2 leads to serious defects in skin development. These mice perished within a day of being born. "They basically became dehydrated and died because their skin couldn't keep water and fluid in," said Carstens. This flaw is called an epithelial barrier defect, found in human disorders and includes the skin, gut, and lungs. Carstens hopes that more study of Esrp1 and Esrp2, particularly the proteins isoforms they control, will uncover clues to how these defects occur and how they might be corrected. Carsten also hopes Esrp1-knockout mice will prove to be a valuable new model animal for studying cleft lip, adding: "There have been many knockout mouse models of cleft palate, but very few of cleft lip, which is actually the more common defect in humans." Abstract DOI: http://dx.doi.org/10.7554/eLife.08954.001 See more at: http://elifesciences.org/content/4/e08954.full#sthash.e8r8JHqz.dpuf The lead authors of this work were postdoctoral fellows Thomas W. Bebee, from Penn, who generated and characterized the knockout mice, who along with bioinformatic analysis by Juw Won Park, from UCLA, profiled the global molecular changes in the knockout mice. Other coauthors are Katherine Sheridan, Claude Warzecha, Benjamin Cieply, and Alex Rohacek, all from Penn. Funding was provided by the National Institutes of Health (R01 GM088809, R56 AR066741, R56 DE024749, P30 AR057217, P30 AR050950, P30 DK050306, T32DK700638, F32DK098917, R01 GM088342, R01 GM105431), and a research fellowship to Yi Xing from the Alfred P. Sloan Foundation. Penn Medicine is one of the world's leading academic medical centers, dedicated to the related missions of medical education, biomedical research, and excellence in patient care. Penn Medicine consists of the Raymond and Ruth Perelman School of Medicine at the University of Pennsylvania (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System, which together form a $4.9 billion enterprise. The Perelman School of Medicine has been ranked among the top five medical schools in the United States for the past 17 years, according to U.S. News & World Report's survey of research-oriented medical schools. The School is consistently among the nation's top recipients of funding from the National Institutes of Health, with $409 million awarded in the 2014 fiscal year. The University of Pennsylvania Health System's patient care facilities include: The Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center -- which are recognized as one of the nation's top "Honor Roll" hospitals by U.S. News & World Report -- Chester County Hospital; Lancaster General Health; Penn Wissahickon Hospice; and Pennsylvania Hospital -- the nation's first hospital, founded in 1751. Additional affiliated inpatient care facilities and services throughout the Philadelphia region include Chestnut Hill Hospital and Good Shepherd Penn Partners, a partnership between Good Shepherd Rehabilitation Network and Penn Medicine.
|
Sep 21, 2015 Fetal Timeline Maternal Timeline News News Archive
|
||||||||||||||||||||||||||||