|
|
Restoring vision using pluripotent stem cells
Research has succeeded in producing photoreceptors from human embryonic stem cells.
Age-related macular degeneration (ARMD) may be treatable by transplanting photoreceptors produced from differentiated stem cells. These findings by Gilbert Bernier PhD, Assistant Professor at the University of Montreal and its affiliate Maisonneuve-Rosemont Hospital, are published in the journal Development, October 15, 2015 issue.
ARMD is a common eye problem caused by the loss of cones. Bernier's team has developed a highly effective laboratory technique for producing light sensitive cone retina cells from human embryonic stem cells.
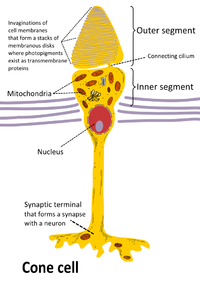
The retina has two types of photoreceptor cells, rods and cones - both specialized neurons.
Rods are numerous - about 120 million - and are basically modified cilia sensitive to movement.
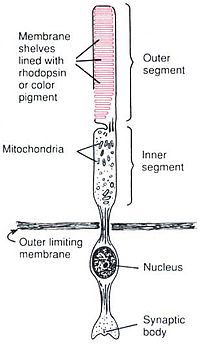
The inner segment of both rod and cone are full of mitochondria and provide ATP (energy) to the cell.
|
|
| 6 to 7 million cones concentrate at the very center of the macula in the "fovea centralis" - a rod-free, mere 0.3 mm diameter in the retina center.
Cones contain three different classes of photopsins reacting to photons of different wavelengths. Their shape calculates color based on the angle at which a photon is intercepted.
|
|
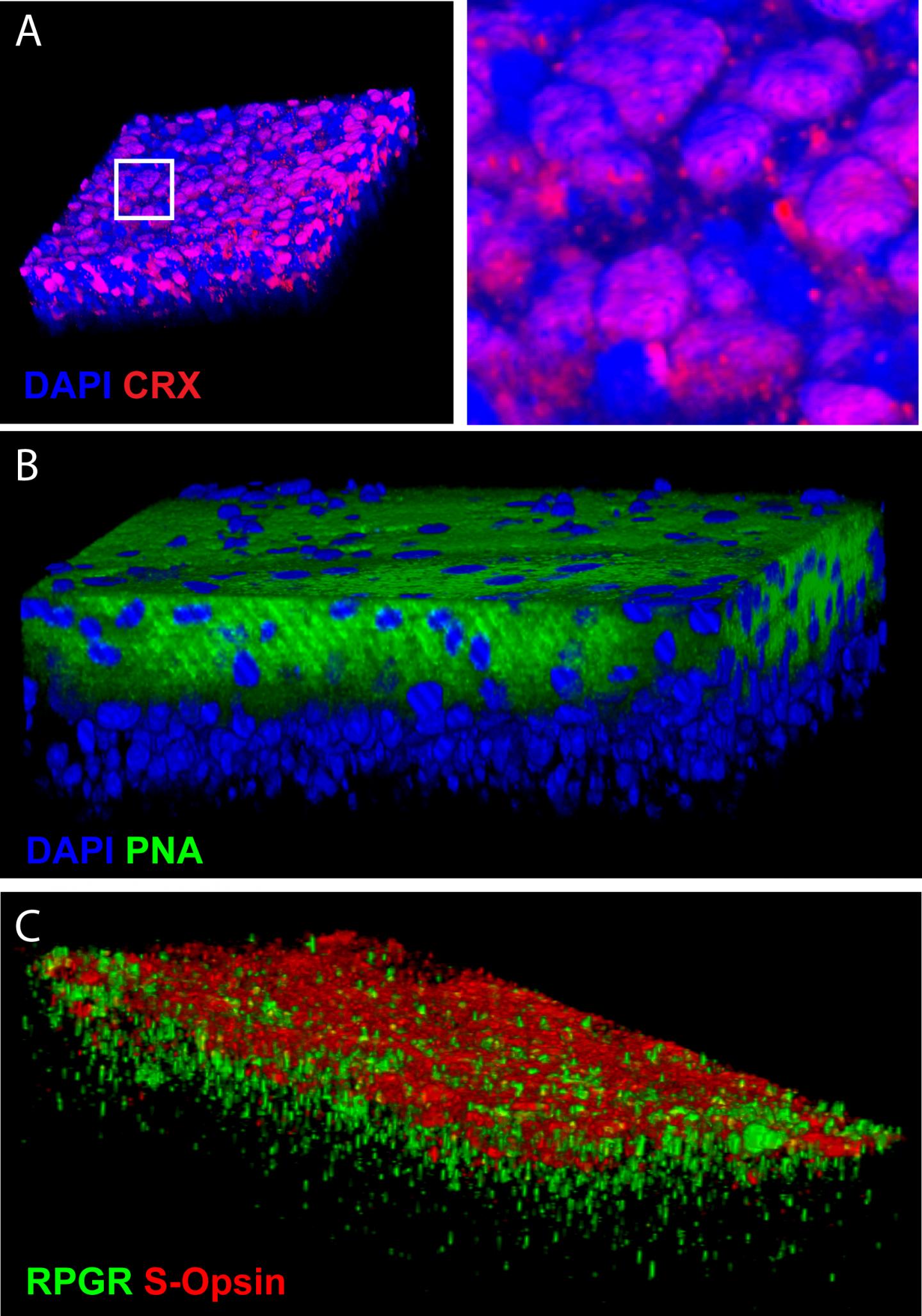
"Our method has the capacity to differentiate 80% of stem cells into pure cones. Within 45 days, the cones grew spontaneously forming organized retinal tissue 150 microns thick — a feat never achieved before."
Gilbert Bernier PhD, Professor, University of Montreal, Canada.
To verify the technique, Bernier injected clusters of retinal cells into the eyes of healthy mice. These transplanted photoreceptors migrated quite naturally into the retina of the host animal.
Bernier: "Cone transplant is a therapeutic solution for retinal pathologies caused by degeneration of photoreceptor cells. To date, it has been difficult to obtain great quantities of human cones." His discovery offers a way to overcome that problem, and offers hope for other currently incurable degenerative eye diseases. "Researchers have been trying to achieve this kind of trial for years. Thanks to our simple and effective approach, any laboratory in the world will now be able to create masses of photoreceptors. Even if there's a long way to go before launching clinical trials, this means in theory we will eventually be able to treat countless patients."
These findings are particularly significant with an improved human life expectancy and anticipated increase in cases of ARMD. ARMD is in fact the greatest cause of blindness amongst people over the age of 50, affecting millions of people worldwide. Nearly one in four people over 80, experiences accelerated aging of the retina. People with ARMD gradually lose their perception of color and detail to the point they can no longer read, write, watch television or even recognize a face.
ARMD is due to the degeneration of the macula, the central part of the retina where cone cells are concentrated. Repair or elimination of worn out rod and cone cells would otherwise occur in the Retinal Pigment Epithelium (RPE). However, as we are born with a fixed number of cones, these cells are not genetically destined to be replaced.
"Differentiating RPE cells is quite easy. But in order to undertake a complete therapy, we need neuronal tissue that links RPE cells to cones. That is much more complex. During my post-doc at the Max-Planck Institute in Germany, I developed an idea that a natural molecule must exist capable of forcing embryonic stem cells into becoming cones."
Bioinformatic analysis led Bernier to predict the existence of a mysterious protein — COCO — as a possible "recombinational" molecule expressed by photoreceptors during eye development.
In 2001, he launched his laboratory at Maisonneuve-Rosemont Hospital and immediately isolated a COCO-like molecule. But it took years of research to demystify the molecular pathways and learn how all photoreceptor development worked.
His latest research reveals that to create cones, COCO systematically blocks all signalling pathways leading to differentiation in any other eye retinal cell, acting much like a master on/off switch. Unraveling this molecular process led Bernier to ultimately produce S-cones.
All receptors contain the protein photopsin, with variations caused by differences in wavelengths absorbed. Humans normally have three types of cones. The L-cone responds the most to light of long wavelengths, peaking at about 560nm. The M-cone responds the most to light of medium-wavelength, peaking at 530nm. The S-cone responds to the shortest-wavelength light, peaking at 420nm. The three peak wavelengths, depend on each individual. Signals received from the three cone types allow the brain to perceive a continuous range of colors.
S-cones, are found in even the most primitive organisms.
Professor Bernier's findings could possiblby enable remodelling the human retina with induced pluripotent stem cells converted into S-cone cells. Such a possibility could even allow for the use of a patient's own tissues to be part of their therapy.
Abstract
Cone photoreceptors are required for color discrimination and high-resolution central vision and are lost in macular degenerations, cone and cone/rod dystrophies. Cone transplantation could represent a therapeutic solution. However, an abundant source of human cones remains difficult to obtain. Work performed in model organisms suggests that anterior neural cell fate is induced ‘by default' if BMP, TGFβ and Wnt activities are blocked, and that photoreceptor genesis operates through an S-cone default pathway. We report here that Coco (Dand5), a member of the Cerberus gene family, is expressed in the developing and adult mouse retina. Upon exposure to recombinant COCO, human embryonic stem cells (hESCs) differentiated into S-cone photoreceptors, developed an inner segment-like protrusion, and could degrade cGMP when exposed to light. Addition of thyroid hormone resulted in a transition from a unique S-cone population toward a mixed M/S-cone population. When cultured at confluence for a prolonged period of time, COCO-exposed hESCs spontaneously developed into a cellular sheet composed of polarized cone photoreceptors. COCO showed dose-dependent and synergistic activity with IGF1 at blocking BMP/TGFβ/Wnt signaling, while its cone-inducing activity was blocked in a dose-dependent manner by exposure to BMP, TGFβ or Wnt-related proteins. Our work thus provides a unique platform to produce human cones for developmental, biochemical and therapeutic studies and supports the hypothesis that photoreceptor differentiation operates through an S-cone default pathway during human retinal development.
About this study:
Shufeng Zhou, Anthony Flamier, Mohamed Abdouh, Nicolas Tétreault, Andrea Barabino, Shashi Wadhwa and Gilbert Bernier published "Differentiation of human embryonic stem cells into cone photoreceptors through simultaneous inhibition of BMP, TGFβ and Wnt signaling" in Development on October 6, 2015.
Gilbert Bernier is director of the Stem Cell and Developmental Biology Laboratory at Maisonneuve-Rosemont Hospital and a professor with the Department of Neuroscience and the Department of Opthalmology at the University of Montreal.
This work was supported by grants from the Foundation Fighting Blindness Canada, Turmel Family Foundation for Macular Degeneration Research, Canadian Stem Cell Network, C. Durand Foundation, the GO Foundation, and Natural Science and Engineering Research Council of Canada [grant #250970-2012]. Professor Bernier was supported by the Fonds de recherche du Québec - Santé.
The University of Montreal is officially known as Université de Montréal. Maisonneuve Rosemont Hospital is part of Centre intégré universitaire de santé et de services sociaux de l'Est-de-l'Île-de-Montréal.
Return to top of page
|
|
|
Oct 8, 2015 Fetal Timeline Maternal Timeline News News Archive
Age-related macular degeneration could be treated by transplanting photoreceptors
produced by the directed differentiation of stem cells, thanks to findings published
today by Professor Gilbert Bernier of the University of Montreal and its
affiliated Maisonneuve-Rosemont Hospital.
Image Credit: G. Bernier, Université de Montréal
|
|
| |
|