|
|
Welcome to The Visible Embryo, a comprehensive educational resource on human development from conception to birth.
The Visible Embryo provides visual references for changes in fetal development throughout pregnancy and can be navigated via fetal development or maternal changes.
The National Institutes of Child Health and Human Development awarded Phase I and Phase II Small Business Innovative Research Grants to develop The Visible Embryo. Initally designed to evaluate the internet as a teaching tool for first year medical students, The Visible Embryo is linked to over 600 educational institutions and is viewed by more than one million visitors each month.
Today, The Visible Embryo is linked to over 600 educational institutions and is viewed by more than 1 million visitors each month. The field of early embryology has grown to include the identification of the stem cell as not only critical to organogenesis in the embryo, but equally critical to organ function and repair in the adult human. The identification and understanding of genetic malfunction, inflammatory responses, and the progression in chronic disease, begins with a grounding in primary cellular and systemic functions manifested in the study of the early embryo.

The World Health Organization (WHO) has created a new Web site to help researchers, doctors and patients obtain reliable information on high-quality clinical trials. Now you can go to one website and search all registers to identify clinical trial research underway around the world!

|
|
| Disclaimer: The Visible Embryo web site is provided for your general information only. The information contained on this site should not be treated as a substitute for medical, legal or other professional advice. Neither is The Visible Embryo responsible or liable for the contents of any websites of third parties which are listed on this site. |
|
|

Content protected under a Creative Commons License. Commons License. |
|
| No dirivative works may be made or used for commercial purposes. |
|
|
| |
|
|
 
CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
|
|
|
A gene that prevents Parkinson's disease and dementia
More than half of Parkinson's disease (PD) patients develop progressive disease showing signs of dementia similar to Alzheimer's. However, a research team at the University of Copenhagen, Denmark, has discovered that non-inheritable PD may be due to changes in the immune regulating gene Interferon-beta (IFNβ).
An estimated seven to ten million people worldwide are living with PD, an incurable and progressive disease of the nervous system affecting movement and cognitive ability. However, treatment with IFNβ-gene therapy successfully prevented neural death and disease effects in an experimental animal model of Parkinson's.
The results of that work have just been published in the prestigious scientific journal Cell.
The human brain consists of approximately 100 billion neurons, which coordinate activities in all parts of the body. At Biotech Research and Innovation Centre (BRIC) at the University of Copenhagen, the group of Professor Shohreh Issazadeh-Navikas has discovered that the immune gene IFNβ plays a vital role in keeping neurons healthy.
"We found that IFNβ is essential for neurons' ability to recycle waste proteins. Without IFNβ, the waste proteins accumulate in disease-associated structures called Lewy bodies — in time those neurons die."
Patrick Ejlerskov PhD, Assistant Professor, Biotech Research and Innovation Centre (BRIC), University of Copenhagen, and first author on the report.
The research team found that mice that do not have IFNβ develop Lewy bodies in areas of the brain controlling restoration of memory and movement. As a result, these mice developed disease and clinical signs similar to PD and Dementia with Lewy bodies (DLB).
While hereditary gene mutations have long been known to play a role in familial PD, the BRIC study offers one of the first models for so-called non-familial PD.
Non-familial PD is a form of PD suffered by the majority (90-95%) of patients. Now, according to professor Shohreh Issazadeh-Navikas, the new work opens new possibilities for therapies.
"This is one of the first genes found to cause pathology and clinical features of non-familial PD and DLB, through accumulation of disease-causing proteins.
"IFNβ is independent of gene mutations known from familial PD. When we introduced IFNβ-gene therapy, we could prevent neuronal death and development of disease.
"Our hope is that this new knowledge will enable development of more effective treatment for PD."
Shohreh Issazadeh-Navikas PhD, Professor, Biotech Research and Innovation Centre (BRIC), University of Copenhagen.
Current treatments are effective at improving symptoms of early motor PD, but as the disease progresses, treatment effects are lost. With this incentive, the research team will continue unraveling the molecular mechanisms of how IFNβ protects neurons while preventing movement disorders and dementia.
Abstract Highlights
•Lack of neuronal IFN-β-IFNAR signaling causes brain Lewy body accumulation
•IFN-β deficiency causes late-stage autophagy block and thereby α-synuclein aggregation
•IFN-β promotes neuronal autophagy and α-synuclein clearance
•Ifnb gene therapy prevents dopaminergic neuron loss in a familial PD model
Summary
Neurodegenerative diseases have been linked to inflammation, but whether altered immunomodulation plays a causative role in neurodegeneration is not clear. We show that lack of cytokine interferon-β (IFN-β) signaling causes spontaneous neurodegeneration in the absence of neurodegenerative disease-causing mutant proteins. Mice lacking Ifnb function exhibited motor and cognitive learning impairments with accompanying α-synuclein-containing Lewy bodies in the brain, as well as a reduction in dopaminergic neurons and defective dopamine signaling in the nigrostriatal region. Lack of IFN-β signaling caused defects in neuronal autophagy prior to α-synucleinopathy, which was associated with accumulation of senescent mitochondria. Recombinant IFN-β promoted neurite growth and branching, autophagy flux, and α-synuclein degradation in neurons. In addition, lentiviral IFN-β overexpression prevented dopaminergic neuron loss in a familial Parkinson’s disease model. These results indicate a protective role for IFN-β in neuronal homeostasis and validate Ifnb mutant mice as a model for sporadic Lewy body and Parkinson’s disease dementia.
Return to top of page
|
|
|
Oct 12, 2015 Fetal Timeline Maternal Timeline News News Archive
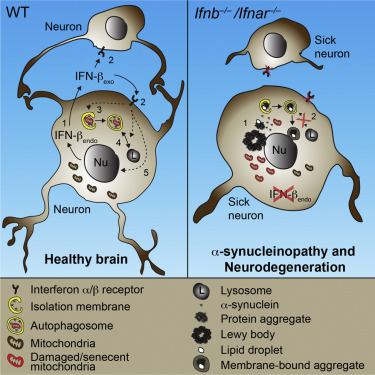
[LEFT] Healthy Neuron with IFNβ— [RIGHT] Sick Neuron with Lewy bodies
Image Credit: Shohreh Issazadeh-Navikas
group,
Biotech Research and Innovation Centre (BRIC), University of Copenhagen
|
|
| |
|



