|
Htt protein controls Rab direction on neurons
We've known for years that the Huntingtin protein (Htt) is responsible for a neurodegenerative disorder that diminishes a person's mental and physical capabilities. Now there is new research that helps explain the root causes of Huntington's disease.
Create Htt protein in the wrong form in the human body, and disturbing symptoms develop. But why mutations in this protein cause disease at all has been an unanswered question.
A new study by University of Buffalo researchers is a step toward understanding this enigma. Published in the journal Human Molecular Genetics, the research shows that Htt controls movement of precious cargo traveling up and down neurons, cells that are the core of all human and animal nervous systems.
You can see videos of vesicle movement along neuronal highways in action. Microscope photos taken by the research team show Htt moving vesciles filled with precious materials called Rab proteins, which they carry over neural highways. Rab proteins are vital to normal cell function.
When scientists lowered the amount of normal Htt, the movement of four different Rab proteins changed, the proteins either slowed down or sped up. Slowing down Rab proteins resulted in traffic jams which blocked other important cargo from traveling to their destinations on neurons.
The research was done in fruit fly larvae, but all four Rab proteins studied are present in human neurons, and the cellular mechanisms at play are expected to be found in people too.
"The problem with the Huntingtin protein is that we know it's linked to Huntington's disease, but we don't have a full understanding of what the protein's normal function is — what purpose it serves in the body, and why problems with it lead to disease.
"Our research begins to unravel the mystery of how Htt is involved in Huntington's disease. If we can better understand the role that Htt plays in the body, inside cells, we can perhaps create therapeutics that address the disease at an early stage, before symptoms become severe."
Shermali Gunawardena PhD, Associate Professor, Biological Sciences, State University of New York at Buffalo (UB) College of Arts and Sciences.
Gunawardena says it's possible that the neural traffic jams her team observed could be contributing to formation of inclusions — dangerous protein aggregates — found in the neurons of Huntington's patients.
Htt as a cell traffic controller
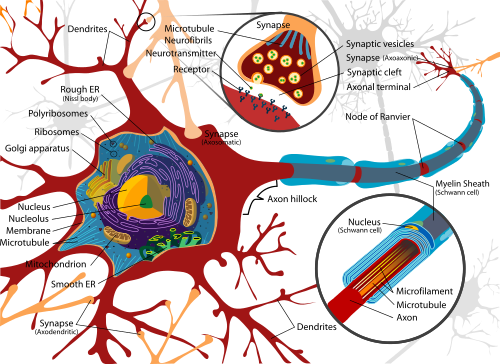
Inside neurons, a network of microtubules serve as a highway system, enabling transport of materials from one part of the cell to another. The vehicles that ferry cargo along these roadways are vesicles — tiny, membrane like structures that enclose or otherwise carry materials ranging from neurotransmitters to chemicals used in cell repair.
The proper functioning of this highway system is needed for the proper functioning of all cells. The new UB study illuminates the role Htt plays in keeping these roadways humming.
The research focused on Htt's influence on Rab proteins, which travel with vesicles along neuronal highways and are thought to be necessary for vesicle formation and movement.
There are 75 known Rab proteins. The study examined 16 Rabs found in both fruit fly and human neurons. When normal levels of Htt were reduced in fruit fly larvae neurons, scientists made the following observations:
-
1) Movement of Rab3 and 19 slowed, along with the vesicles attached to them. Movement slowed for proteins moving both toward and away from the neurons' main cell body.
2) Movement of Rab7 slowed, along with the vesicles attached to it — but only toward the central cell body.
3) Movement of Rab2 sped up, along with the vesicles attached to it — but only away from the central cell body.
Each Rab vesicle transports materials via neurons with functions vital to the health of the nervous system. Therefore, changes in vesicle movement could lead to neurological problems, explains Gunawardena.
"Our research starts to create a picture of how Htt may contribute to disease. If HTT is needed for particular aspects of Rab movement — and the function of certain Rabs — then ensuring this pathway is not disrupted could provide a new therapeutic avenue for a disease that is now untreatable."
Shermali Gunawardena PhD
In addition to Rabs 3, 19, 7 and 2, the movement of a fifth Rab protein was also affected — but researchers are still analyzing findings related to this fifth protein, adds Gunawardena. Other Rab proteins studied were not impacted by a reduction in Htt.
Abstract
Loss of huntingtin (HTT), the Huntington's disease (HD) protein, was previously shown to cause axonal transport defects. Within axons, HTT can associate with kinesin-1 and dynein motors either directly or via accessory proteins for bi-directional movement. However, the composition of the vesicle-motor complex that contains HTT during axonal transport is unknown. Here we analyze the in vivo movement of 16 Rab GTPases within Drosophila larval axons and show that HTT differentially influences the movement of a particular sub-set of these Rab-containing vesicles. While reduction of HTT perturbed the bi-directional motility of Rab3 and Rab19-containing vesicles, only the retrograde motility of Rab7-containing vesicles was disrupted with reduction of HTT. Interestingly, reduction of HTT stimulated the anterograde motility of Rab2-containing vesicles. Simultaneous dual-view imaging revealed that HTT and Rab2, 7 or 19 move together during axonal transport. Collectively, our findings indicate that HTT likely influences the motility of different Rab-containing vesicles and Rab-mediated functions. These findings have important implications for our understanding of the complex role HTT plays within neurons normally, which when disrupted may lead to neuronal death and disease.
The study was funded by the National Institute of Neurological Disorders and Stroke and in part by the John R. Oishei Foundation.
Return to top of page
|