|
|||||||||||||||
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
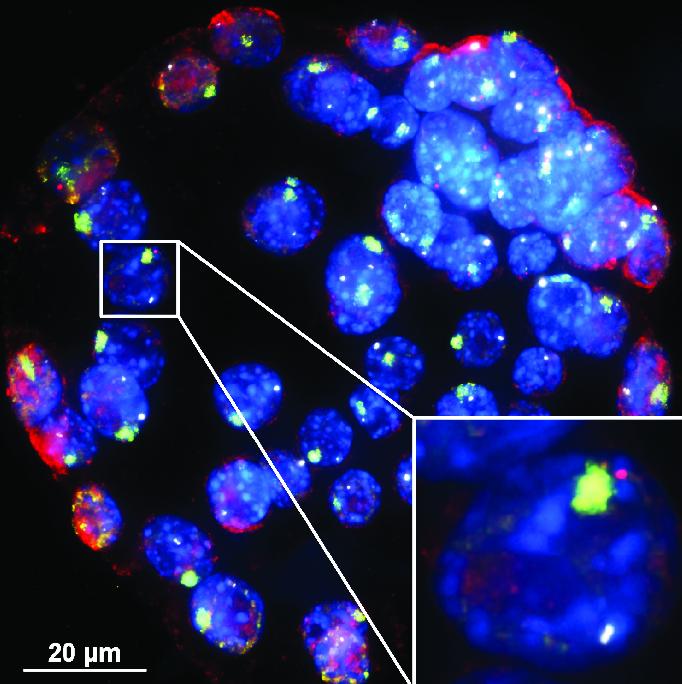
How RNA regulates sex-linked disorders Boys have an X and a Y chromosome inside each cell. Girls have two X's. That small difference explains why boys are more at risk for disorders, from autism to hemophilia, linked to gene defects on their one X chromosome. We may now know how parts of that X can be silenced — to better treat X-linked diseases. Most girls who carry the same defect on one of their X chromosomes as a boy with the same X chromosome, won't get sick. This is because their other X chromosome takes over. It becomes the "back-up" X whose genes function, while the other X is "silenced". A new gene discovery helps scientists explain how this happens. In a new paper in Nature Communications, scientists from the University of Michigan Medical School show that in female cells — not male cells — tiny amounts of RNA are made that silence the defective X chromosome. They call it 'Xist Activating RNA' — or XistAR. Back in high school biology, you probably learned that RNAs make proteins in cells. But XistAR is special. It belongs to a class of RNAs called long noncoding RNAs — or lncRNAs. These RNAs don't make proteins, they control how genetic code is read.
Genes can be 'read' in both a forward and backward direction which increases their functionality. These molecules are held together by electro-magnetic fields, and although they have the same molecular formula and sometimes appear in the same sequence, they can have a different arrangement of atoms and so have different properties.
To prove their discovery, the team developed a new test to find backward read RNA strands, which cells make in much smaller amounts than forward-read RNA strands. The test can now search through the genome for more examples of rare lncRNAs — made in the backward or 'antisense' direction. Experimenting in mice, scientists could follow what happens when they stop cells from making XistAR. Without XistAR, cells couldn't silence one of the two female X chromosomes which resulted in too many X-chromosome gene products. If this discovery translates to humans — and XistAR seems to be present in humans — Kalantry believes it may provide a convenient handle to manipulate otherwise defective genes on X chromosomes. For example, it might be possible to boost proteins inaccessible in defective genes carried in some male X chromosomes. Kalantry's team is now looking for factors to control XistAR.
Meanwhile, Kalantry says, understanding how antisense lncRNAs work in an "epigenetic" way — as an outside force controlling what gene gets read or not — applies far beyond the X-chromosome. Calico cats and autism Kalantry, an assistant professor in the U-M Department of Human Genetics, keeps a portrait of a calico cat on his office wall to remind him of the power of the X-chromosome inactivation. But in humans, the effects of in-activating a "healthy" X chromosome more often than an "unhealthy" X — can really change a girl's life. For example, Rett syndrome is an autism-like disorder occuring only in girls. It occurs when a mutated gene on one of her two X chromosomes is 'turned on'. Autism is heavily linked to genetic problems on the X-chromosome. This explains why boys with only one X chromosome are four times more likely than girls to be on the autistic spectrum. What Happens Next?
Kalantry: "The control of genes by lncRNAs is often via epigenetic influences, now realized to occur in a wide variety of contexts from normal physiology to disease. On a fundamental level, lncRNAs reverse the central dogma of DNA begetting RNA, which then make proteins. Techniques we've developed facilitate the discovery of rare RNA species in a cell. Such RNAs, to date, have been missed by high-throughput gene sequencing although essential to cell function." Abstract The research was funded by the National Institutes of Health, including a New Innovator Award to Kalantry (OD008646). Three post-doctoral fellows share first authorship on the study: Mrinal Kumar Sarkar, Ph.D., Srimonta Gayen, Ph.D., and Surinder Kumar, Ph.D. Reference: Nature Communications, DOI: 10.1038/ncomms9564, http://www.nature.com/ncomms/2015/151019/ncomms9564/full/ncomms9564.html |
Oct 23, 2015 Fetal Timeline Maternal Timeline News News Archive
|
||||||||||||||||||||||||||||