|
|||||||||||||||
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
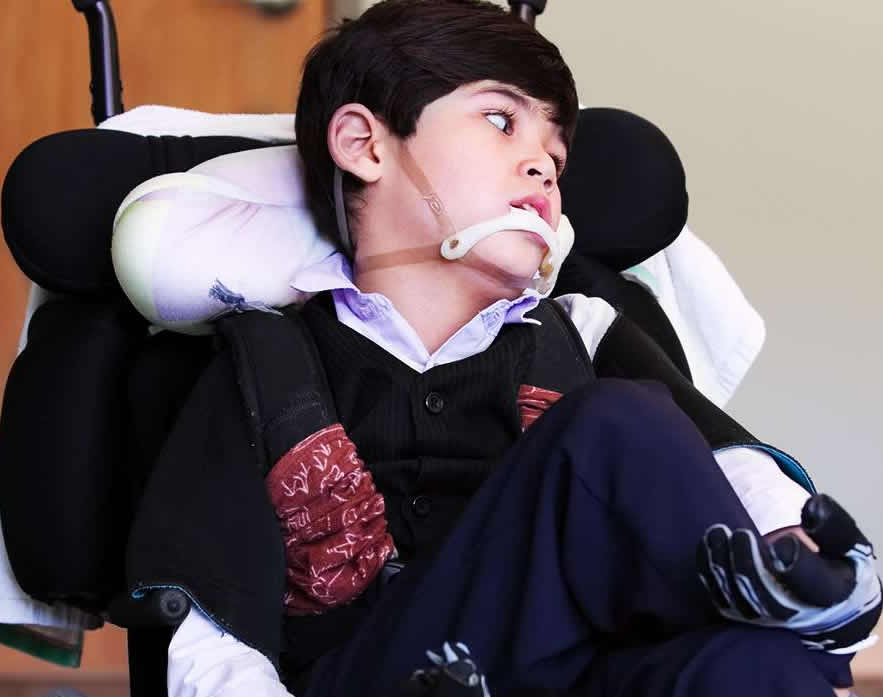
Batten disease may benefit from gene therapy Early symptoms of Batten disease, a lysosomal storage disorder, may include vision loss, subtle personality and behavior changes, slow learning, clumsiness or stumbling. Eventually, the child becomes blind, bedridden and demented. Death usually comes within the first decade of life. There are no effective treatments. "Our study opens up the possibility of a one-and-done treatment for this form of Batten disease," said Beverly Davidson, Ph.D., director of the Raymond G. Perelman Center for Cellular and Molecular Therapeutics at Children's Hospital of Philadelphia and the senior author of the study. Working with scientists at the University of Missouri, Columbia, Davidson's team focused on the late infantile form of the disease that starts in children 2 to 4 years of age. It is most often caused by mutations in the gene for soluble lysosomal enzyme tripeptidyl peptidase 1 (TPP1) — an enzyme which degrades proteins.
Symptoms including problems with movement, pupil dilation and making decisions were delayed or in some cases did not occur. The treatment, however, did not fully improve a dogs' vision suggesting that delivery to the eye itself may be necessary as well. When the brains were inspected, the treatment had reduced damage normally caused by the disease. In comparison with untreated dogs, treated dogs had less reactive glial cells or stored lipofuscins — fatty deposits that are hallmarks of Batten disease and similar disorders. "Dr. Davidson and her team undertook a highly innovative approach for Batten disease gene therapy," said Jill Morris, Ph.D., program director at NIH's National Institute of Neurological Disorders and Stroke. "These results open up a promising path toward developing long-lasting treatments for Batten disease and similar lysosomal storage disorders."
Initial experiments showed that it was important to treat the dogs with an immunosuppressant — mycophenolate mofetil — before injecting the TPP1 gene. The immunosuppressant prevented the body from producing antibodies to clear TPP1 from the cerebrospinal fluid. "We saw profound effects from the gene therapy that, summed up, improved the dogs' health. We certainly hope that this approach will provide children suffering from this disorder similar benefits," said Dr. Davidson. Abstract References: Katz, Tecedor, Chen et al. "AAV gene transfer delays disease onset in a TPP1-deficient canine model of the late infantile form of Batten disease," Science Translational Medicine, November 11, 2015. DOI: 10.10.1126/scitranslmed.aac6191 For more information, visit: http://www.ninds.nih.gov The NINDS is the nation's leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease. NEI leads the federal government's research on the visual system and eye diseases. NEI supports basic and clinical science programs that result in the development of sight-saving treatments. For more information, visit http://www.nei.nih.gov. About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit http://www.nih.gov. |
Nov 13, 2015 Fetal Timeline Maternal Timeline News News Archive
|
||||||||||||||||||||||||||||