|
|||||||||||||||
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
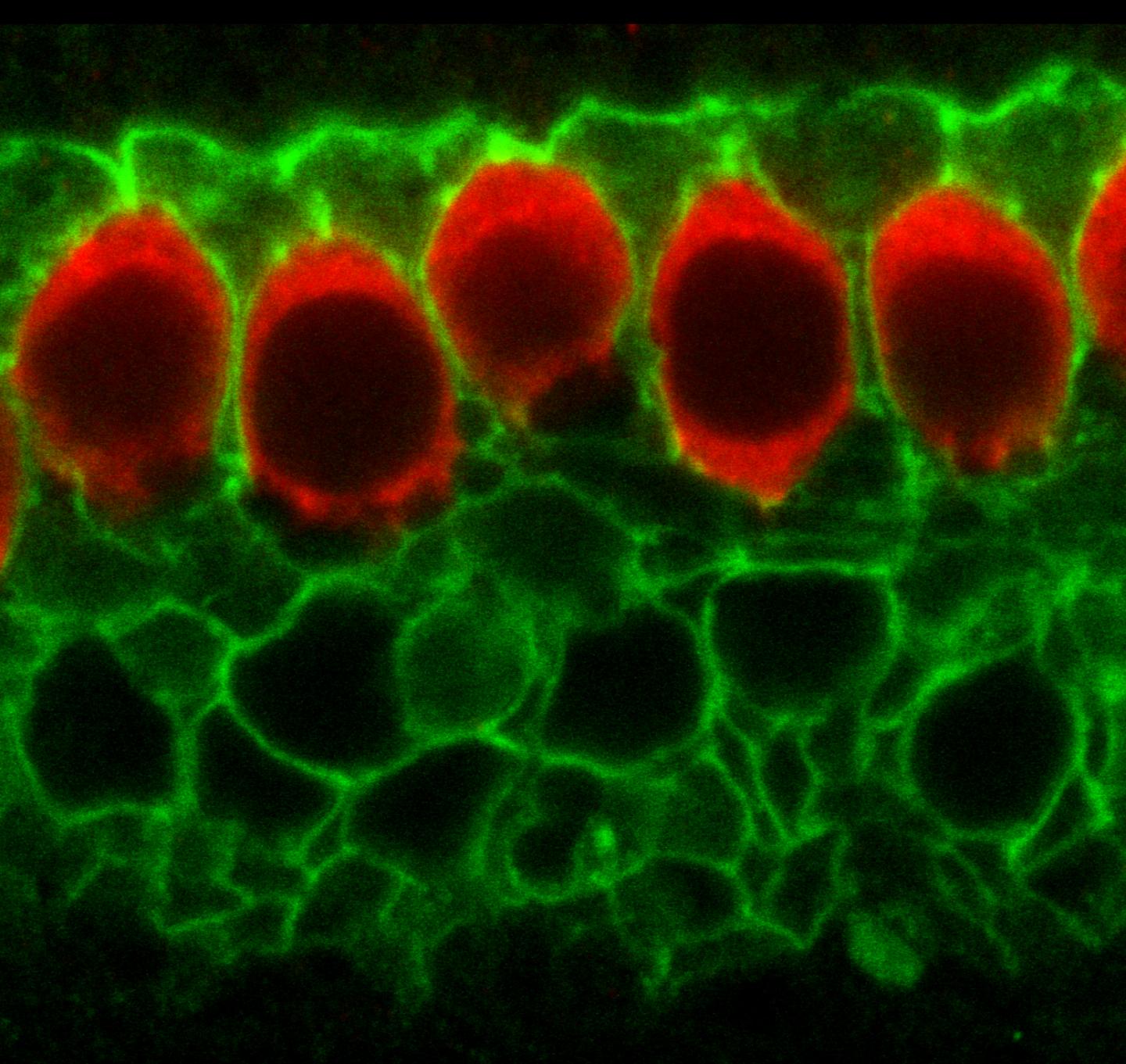
How cells in the developing ear 'practice' hearing Before the fluid of the middle ear drains and sound waves penetrate those cells for the first time, inner ear cells of newborn rodents practice for their big debut. Researchers at Johns Hopkins report they have figured out the molecular chain of events that enables cells to make "sounds" on their own, essentially "practicing" how to process sound in the world around them. Describing their experiments in the December edition of the journal Cell, scientists show how hair cells in the inner ear can be activated in the absence of sound. "The multistep process we uncovered reminds me of a Rube Goldberg invention. Cells in the inner ear exploit a system used for fluid secretion in other organs to simulate the effect of sound before hearing begins, preparing them for the real deal," says Dwight Bergles PhD, professor of neuroscience at the Johns Hopkins University School of Medicine, in a reference to Rube Goldberg's construction of complicated gadgets to perform seemingly simple tasks.
Scientists, Bergles says, already knew that hair cells and nearby "supporting" cells in the developing inner ear show synchronous bursts of activity triggered by release of the chemical ATP — also used as a potent communication signal. This activity is then conveyed to the brain in the same way that sound-evoked information is, leading to burst firing of neurons in different auditory centers of the brain. To find out, Bergles and his team honed in on biochemical elements of the system and found that chloride ion channels in supporting cells appeared crucial. They knew that ATP triggers a rise in calcium levels inside supporting cells, so they guessed that calcium was the signal for a calcium-activated chloride channel to open.
Continued biochemical testing, combined with electrical recordings and imaging of calcium changes in the inner ears of mice, helped the team piece together a full chain of events: (1) supporting cells release ATP, leading to self-stimulation of their own ATP receptors, (2) this triggers an increase in calcium levels inside the cells, (3) a rise in calcium opens the TMEM16A channels to let chloride out, (4) which also drags potassium ions and water out, (5) the potassium released during these events activates hair cells which (6) stimulate nerve cells to which they have formed weak connections. It's this increase in calcium and release of chloride and potassium that activates hair cell sto vibrate and trip nerve cells, says Bergles, which helps the brain make sense of sound.
When the brain receives a signal from hair cells near the entrance of the inner ear, Bergles says, it "interprets" a high-pitched sound; when the signal comes from farther in, it "interprets" a deeper sound. Bergles: "There's a beauty to this seemingly overly complex process. It uses the capabilities of the cells in a novel way to trigger nerve cell activity. We think this helps establish and refine the connections between ear and brain so that the animal can properly hear sounds as soon as exposed to them." Researchers surmise what mice hear based on activity they record in the auditory centers of their brains. Bergles believes "sounds" might be perceived as single tones played in succession, something like tests of an emergency response system. Comparing the training of the ear to what a batting machine is to a baseball player: "The machines don't have all the richness and unpredictability of a pitcher throwing a ball, but they nevertheless help players prepare for the big event."
Abstract Highlights Other authors of the report include Han Chin Wang, Chun-Chieh Lin, Rocky Cheung, YingXin Zhang-Hooks and Amit Agarwal of the Johns Hopkins University School of Medicine; Graham Ellis-Davies of the Icahn School of Medicine at Mount Sinai; and Jason Rock of the University of California, San Francisco. This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NS050274, NS06972), the National Institute of Mental Health (MH084020), the National Institute on Deafness and other Communication Disorders (DC008860), and the National Institute of General Medical Sciences (GM053395). |
Dec 1, 2015 Fetal Timeline Maternal Timeline News News Archive
|
||||||||||||||||||||||||||||