|
|||||||||||||||
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
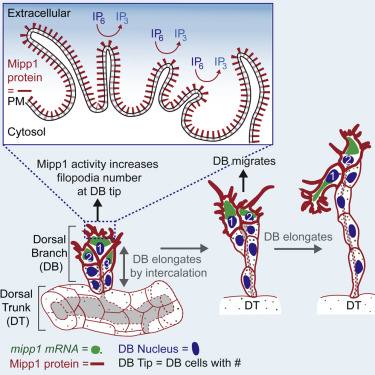
Cells 'reach out' to build trachea Fruit fly windpipes are more like our human blood vessels than entryways to the lungs. According to Deborah Andrew PhD, professor of cell biology at the Johns Hopkins University School of Medicine: "Fruit flies don't have blood to bring oxygen to their cells. Instead, the tubes of the windpipe, or trachea, branch out repeatedly, getting thinner and thinner — like the tiny capillary blood vessels throughout our bodies — so that oxygen can diffuse directly from the trachea into nearby tissue." A summary of this research, which has implications for understanding normal and abnormal development in human and animal tissues, is published online in the journal Cell Reports. Fruit flies are a research animal favorite among biologists because their genes and chemistry are relatively easy to manipulate. It also helps that they easily and quickly breed to improve their status as a model animal to study. As evolution highly conserves key biological events, what is learned from fruit flies may shed light on development in other species, including humans. Andrew points out that the two major ducts of the embryonic fruit fly trachea run parallel to each other along the length of the embryo body. From these wide "dorsal trunks," several thinner branches split off and grow toward the top of the embryo where they meet and merge midline, forming a contiguous network.
"A few years ago, we discovered that in developing fly embryos, the protein Mipp1 is controlled by a master regulator gene orchestrating all of tracheal development," says Yim Ling Cheng, Ph.D., a cell biology postdoctoral fellow at the Johns Hopkins University School of Medicine, and first author. Researchers had known that Mipp1 is an enzyme responsible for turning off chemical messenger molecules IP6 into IP3 ¬ by breaking off three of the phosphate groups. But, how is Mipp1 doing this. By tracking Mipp1, they found the protein is located throughout the developing fruitfly, but soon becomes concentrated in the top pair of cells in the three-cell-high dorsal branches just before elongation. Those are the cells that grow filopodia. And, when there was too much Mipp1, the research team saw too many filopodia. Too little Mipp1 resulted in too few filopodia and branches that were slow to elongate. Wondering if Mipp1's presence in the top cells was the cause or result of a cells' position, researchers genetically manipulated the flies so that dorsal branch cells turned on the Mipp1 gene at random.
Abstract Highlights This work was supported by grants from the National Institute of Dental and Craniofacial Research (RO1 DE012873, F31 DE021285). |
Dec 2, 2015 Fetal Timeline Maternal Timeline News News Archive
|
||||||||||||||||||||||||||||