|
|
Stem cells likely safe for use in regenerative medicine
By transferring human pluripotent stem cells (hPSCs) into the gastrula stage of mouse embryos, and with their subsequent safe incorporation and development into normal mice, Cambridge researchers have now performed the strongest experiment to date supporting stem cells safe use. A breakthrough discovery for regenerative medicine.
Previous attempts at getting human pluripotent stem cells to incorporate into mouse embryos have not been entirely sucessful, as cells failed to proliferate or distribute themselves, and sometimes produced tumors.
But now, in research funded by the British Heart Foundation, Victoria Mascetti PhD, and Professor Roger Pedersen, both of the Department of Surgery and the British Heart Foundation Centre of Regenerative Medicine, University of Cambridge, Cambridge, Uk, show it is possible to successfully transplant human pluripotent stem cells into mouse embryos and grow normal mice.
Mascetti's breakthrough with her new research is to demonstrate human pluripotent stem cells matched to a later stage of mouse embryo development - do well.
The findings are published in the journal Cell Stem Cell.
Human pluripotent stem cells used in regenerative medicine for biomedical research come from two sources: (1) embryonic stem cells, derived from fertilized egg cells discarded from IVF procedures; and (2) induced pluripotent stem cells, derived from skin cells that were 'reset' to their original, pluripotent form. The hope is that stem cells will aid in the treatment of devastating conditions affecting organs and tissues — particularly in those tissues with very poor regenerative capacity such as the heart, brain and pancreas. Scientists have been confronted up until now by poor results in a number of stem cell experiments revealing that stem cells were unable to proliferate or distribute themselves, and sometimes produced tumors.
But this latest research from Cambridge suggests the timing of transplantation may be the key.
The best way to test how well stem cells will incorporate into the body is to transplant them into early-stage mouse embryos and see how they affect an embryo's development. This procedure cannot be done ethically in humans, so scientists use mouse embryos as the gold standard for transplantation. This gold standard was developed at Cambridge University in the 1980s. It currently involves putting stem cells into a mouse blastocyst, a hollow ball of cells formed from developing embryonic mouse cells around 3 to 3.5 days after fertilization.
Mascetti feels previous failed transplantation attempts were possibly due to mismatches between human stem cells and mouse developmental stages.
Stem cells in the Mascetti/Pedersen research were transplanted into the mouse embryo in a later stage known as gastrulation a process which occurs 3.5 to 4.5 days following fertilization. Once transplanted into the gastrula stage, the stem cells grew and proliferated normally, integrating into the embryo and distributing themselves correctly across the three developing cell types and later into developing tissues.
"Our study provides strong evidence to suggest that human stem cells will develop in a normal, and importantly, safe way. This could be the news that the field of regenerative medicine has been waiting for.
"Stem cells hold great promise for treating serious conditions such as heart disease and Parkinson's disease, but until now there has been a big question mark over how safe and effective they will be."
Roger Pedersen PhD, Professor, Anne McLaren Laboratory for Regenerative Medicine, University of Cambridge, United Kingdom
Mascetti adds: "Our finding that human stem cells integrate and develop normally in the mouse embryo will allow us to study aspects of human development during a window in time that would otherwise be inaccessible."
"These results substantially strengthen the view that induced pluripotent stem cells from adult tissue are suitable for use in regenerative medicine — for example in attempts to repair damaged heart muscle after a heart attack.
"The Cambridge team has shown definitively that when stem cells are introduced into early mouse embryos under the right conditions, they multiply and contribute in the correct way to all the cell types that are formed as the embryo develops."
Jeremy Pearson PhD, Associate Medical Director, British Heart Foundation.
Abstract Highlights
• hiPSCs and hESCs form human-mouse interspecies chimeras with high efficiency
• hPSCs colonize gastrula-stage embryos in a manner predicted by fate mapping
• Integrated human cells disperse widely and express relevant differentiation markers
• Human-mouse chimeras provide in vivo functional validation of hPSC pluripotency
Summary
Pluripotent stem cells are defined by their capacity to differentiate into all three tissue layers that comprise the body. Chimera formation, generated by stem cell transplantation to the embryo, is a stringent assessment of stem cell pluripotency. However, the ability of human pluripotent stem cells (hPSCs) to form embryonic chimeras remains in question. Here we show using a stage-matching approach that human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells (hESCs) have the capacity to participate in normal mouse development when transplanted into gastrula-stage embryos, providing in vivo functional validation of hPSC pluripotency. hiPSCs and hESCs form interspecies chimeras with high efficiency, colonize the embryo in a manner predicted from classical developmental fate mapping, and differentiate into each of the three primary tissue layers. This faithful recapitulation of tissue-specific fate post-transplantation underscores the functional potential of hPSCs and provides evidence that human-mouse interspecies developmental competency can occur.
Reference
Victoria L Mascetti and Roger A Pedersen. Human-Mouse Chimerism Validates Human Stem Cell Pluripotency. Cell Stem Cell; Dec. 17, 2015
The research was funded by the British Heart Foundation.
About the journal
Stem Cell Reports is the official journal of the ISSCR and is published by Cell Press. The International Society for Stem Cell Research (ISSCR) is a non-profit, scientific membership organization providing a platform for professional and public education and the promotion of rigorous scientific and ethical standards in stem cell research and regenerative medicine.
Return to top of page
|
|
|
Dec 25, 2015 Fetal Timeline Maternal Timeline News News Archive
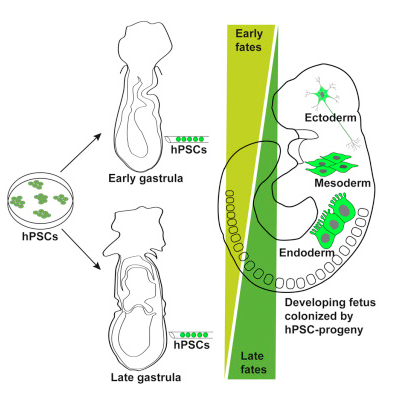
Human Pluripotent Stem Cells, hPSCs, when introduced into the mouse early and late
gastrula embryo, were able to proliferate normally into various normal cell types.
This discovery may lay the foundation for regenerative tissue research using hPSCs.
Image Credit:
Anne McLaren Laboratory for Regenerative Medicine, Cambridge, UK
|
|
| |
|



