|
|||||||||||||||
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
|||||||||||||||||||||||||||
|
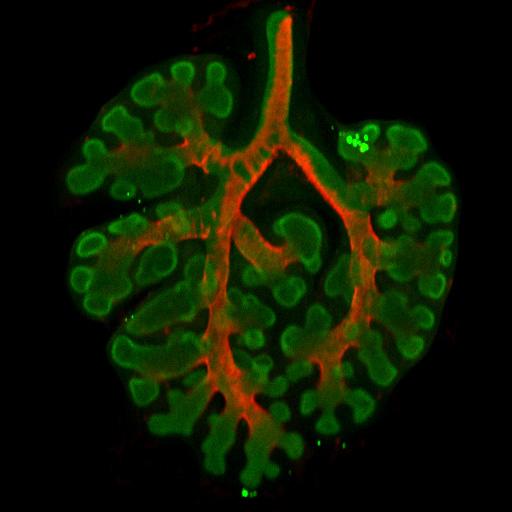
Mechanism for how organs branch found In a series of recent experiments, researchers in the lab of Celeste Nelson PhD, professor of chemical and biological engineering, have found that airway branching in the developing lung is regulated in part by mechanical forces experienced by embryonic tissues. This insight expands the standard theory that airway branching is controlled by a closed genetic program, hardwired in DNA. "Our work indicates that physical forces can determine the locations where new branches form within the developing lung" says Victor Varner, a post-doctoral scientist in chemical and biological engineering, and lead author on one of two papers recently published on the subject. The results help us understand developmental disorders in babies and have implications for treating growth disorders found in cancer, and the potential for developing lab-grown replacements for other human organs.
The lung's array of twisting branches may appear to be random, but follow a consistent pattern. Previous work in this field has suggested lung pattern is determined by distribution of biochemical molecules within the embryonic lung tissue — following one particular molecule, FGF 10 (fibroblast growth factor), thought to direct development and branching in airways. Varner and Nelson wondered if biochemical signals were the only way to control airway branching. In one experiment, they dissected a small piece of lung tissue from an early mouse embryo and separated the developing airways from the adjacent cells expressing FGF10, thereby disrupting the biochemical pre-pattern thought to control the formation of new branches. These fragments of developing airway were then cultured in a three-dimensional gel and, remarkably, continued to grow and branch in the laboratory. "Once we'd disrupted the pre-pattern growth factors, given my background as a mechanical engineer, I wondered if a physical mechanism might controll the formation of new branches in culture,"added Victor Varner, a post-doctoral student in chemical and biological engineering in Nelson's laboratory. The article on the study was published on July 28, 2015, in the Proceedings of the National Academy of Sciences or PNAS Varner said the researchers determined that a mechanical instability, similar to the buckling of a beam or column under compressive force, was generated within the growing airway and that this instability dictated where new branches formed along the tissue.
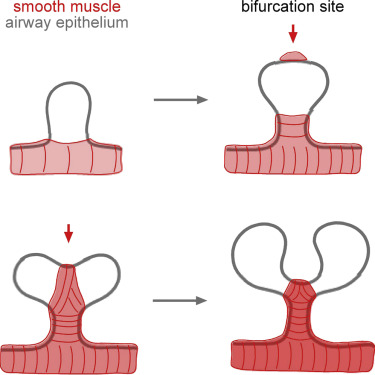
For their next experiment, the researchers want to examine how mechanical stresses affect branching in the intact embryonic lung, a far more complex environment than a simplified 3D culture. Eventually, they hope to find how physical forces contribute to underlying congenital branching defects. In a related but separate study, Nelson's lab scientists also determined that bifurcations in the developing lung are sculpted by a layer of smooth muscle wrapping around the airways. This work, published Sept. 28, 2015, in Developmental Cell Nelson and colleagues found smooth muscle cells accumulate in the cleft at the tip of newly forming bifurcations — pulling the tube into two new daughter branches. Investigating further, researchers found that when smooth muscle growth was disrupted, airways no longer split into two branches. According to Nelson, these results highlight the role of mechanical forces in lung development while also revealing that smooth muscle "acts like a girdle forcing developing airways into shape."
Abstract In addition to Nelson and Varner, the authors of the PNAS article included: James Gleghorn, a postdoctoral researcher in chemical and biological engineering at Princeton; Erin Miller and Derek Radisky of the Mayo Clinic Cancer Center. The research was supported in part by the National Institutes of Health, the National Science Foundation, the David and Lucile Packard Foundation, the Alfred P. Sloan Foundation, and the Camille and Henry Dreyfus Foundation. Besides Nelson and Varner, the authors of the Developmental Cell article included: Hye Young Kim and Mei-Fong Pang, post-doctoral researchers in chemical and biological engineering at Princeton; Lisa Kojima, an undergraduate student in chemical and biological engineering; Erin Miller and Derek Radisky of the Mayo Clinic Cancer Center. The work was supported in part by the National Institutes of Health, the National Science Foundation, the David and Lucile Packard Foundation, the Alfred P. Sloan Foundation, the Camille and Henry Dreyfus Foundation, and Susan G. Komen for the Cure.
|
Jan 4, 2016 Fetal Timeline Maternal Timeline News News Archive
|
|||||||||||||||||||||||||||