|
|||||||||||||||
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
|||||||||||||||||||||||||||
|
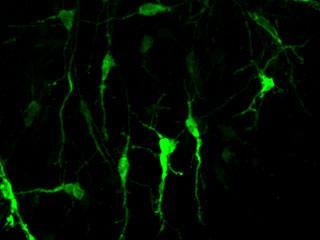
Epigenetic change leads to cerebellum circuitry Now, a team of researchers at The Rockefeller University is describing an epigenetic mechanism underlying development of the cerebellum — the portion of the brain allowing us to learn and execute complex thought. Epigenetics are those environmental influences on our DNA, not inherent in DNA code, but affecting how our genes are able to express proteins.
Epigenetic pathway genes modify chromatin, which is the protein package condensing DNA to fit into a cell. Alterations to chromatin can affect how genes are available to be translated into proteins. Further investigation revealed one of these epigenetic regulators was affecting ion channels as they transmit electrochemical signals across synapses — or gaps between neurons. 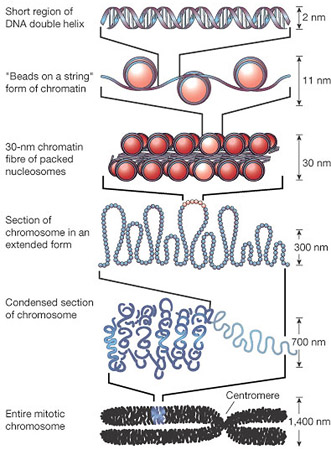 Chromatin proteins condense DNA to fit within a cell. Image Credit: Encyclopedia of Science, The Worlds of David Darling The cerebellum is a relatively simple brain structure, yet its function is anything but simple. This part of the brain controls our ability to move and learn new motor skills, which affects our ability to perceive our body's position and motion, and maintain equilibrium. Hatten's research on the developing mammalian brain focused on two crucial stages: (1) the birth of neurons and (2) the migration of immature neurons into layers — the basic structure in this part of our brain. After young neurons find their place, they send out branches, called dendrites, to connect to fibers from other parts of the brain and establish ion channels. These ion channel connections make electrical activity possible. Until now, no one had known how genes that control these two processes were activated. Two developments in technology made the current study possible: The second is metagene analysis, a mathematical model developed at the Broad Institute of MIT and Harvard that allows researchers to study large sets of genes and see changes in patterns that would be too difficult to interpret by looking at individual genes. Three investigators from Broad collaborated on the current study. The investigators scrutinized the actions of one particular epigenetic pathway, involving TET enzymes, known to make specific changes to chromatin and switch on gene expression — how genes create proteins. Researchers could now activate these enzymes in embryonic stem cells that had just differentiated into immature granule cells. The result was increased expression of axon guidance and ion channel genes, "just as happens during normal development," Hatten added. These two types of genes are critical for generating synaptic connections. The opposite occurred when the genes for the TET enzymes were shut down. "The cells went through early development, but they didn't extend the dendrites needed to make connections they should have made," she explained.
|
Jan 6, 2016 Fetal Timeline Maternal Timeline News News Archive
|
|||||||||||||||||||||||||||