|
|
Physics attempts to explain why stem cells move
Research has shed light on complex interactions of stem cells and molecular diffusion in brain tissue. The calculations may help explain stem cell differentiation and even the formation of the cortex of our brain.
While researching methods for reconstructing 3D neural tissue and neural pathways in the brain and spinal cord, Richard McMurtrey MD, has devised mathematical calculations to understand how nutrients affect these reconstructions.
Stem cells have very unique responses to varying concentrations of specific molecules, so it is important to understand how nutrient signaling works. Many 3D tissues have been constructed with the hope of making "mini-organs" from a patient's own stem cells, including "mini-brains" used to study neurological diseases. The hope is that one day such organoids will become transplantable into damaged patient tissue and initiate regeneration of our healthy tissue.
The ability to guide and direct stem cell development and function in organoids is an essential aspect of regenerative medical research.
McMurtrey: "I first stumbled upon these ideas when trying to figure out how to obtain exact nutrient concentrations for some 3D tissues composed of neural stem cells. The mathematics involved has always fascinated me, but I was surprised that answering my questions was a lot more difficult than I thought. I felt like I kept going down a rabbit hole trying to find solutions, but eventually figured out the math. I think these ideas really help explain the role of diffusion in brain function and neural development. But of course, there is still much more to learn."
During human development, stem cells near the center of the developing brain will migrate outward to form neurons in the cortex on the outer rim of our brain — a region where our thoughts are formed and processed.
The mathematical work in McMurtrey's paper appear in the Journal of Neural Engineering, and describe growth limitations imposed by diffusion and metabolism. But, they also suggest an underlying physical basis for neural migration.
Once neurons are programmed to migrate to the outer surface, the cortex becomes more convoluted — or wrinkled — creating more neural networks.
One of the unique aspects of 3D models is seeing the results from the implementation of equations on cell metabolism. The work enables any researcher to adapt the models to a specific cell type. Even though the mathematics in deriving these equations is complex, researchers only need to have a working knowledge of algebra to use them. McMurtrey uses what are called analytic or explicit solutions that allow simple parameters to be inserted into formulas in order for different solutions to be found.
His current research focuses on reconstructing brain and spinal cord structures and pathways using synthetic 3D neural tissue made from stem cells, biomaterials, and nanotechnology. McMurtrey hopes to use these models to design artficial tissue implants that could be clinically inserted at sites of damage. He believes engineered tissues could be used for studying development and disease under controlled conditions; or even condition 3D cells to enhance their survival as implants in the body.
"I think we will see that, just as with all other systems in the universe, we cannot fully understand a system as complex as the brain until we understand the mathematics governing its' many fundamental components.
"Whether in the complexity of neural networks, the dynamics of cell signaling, gene expression, the electrical activity of neuronal membranes, or the biomechanics of developing tissues, ultimately physics and engineering have a lot to contribute to solving problems in the human body."
Richard J. McMurtrey MD, Institute of Neural Regeneration & Tissue Engineering, Highland, Utah, United States
Abstract
Diffusion models are important in tissue engineering as they enable an understanding of gas, nutrient, and signaling molecule delivery to cells in cell cultures and tissue constructs. As three-dimensional (3D) tissue constructs become larger, more intricate, and more clinically applicable, it will be essential to understand whether cells are receiving appropriate nutrient delivery and to understand internal dynamics and signaling molecule concentrations throughout the tissue. Diffusion characteristics present a significant limitation in many engineered tissues, particularly for avascular tissues and for cells whose viability, differentiation, or function are affected by concentrations of oxygen and nutrients. This paper seeks to provide analytic solutions for certain cases of steady-state and non-steady-state diffusion and metabolism in basic 3D construct designs (planar, cylindrical, and spherical forms), solutions that would otherwise require mathematical approximations achieved through numerical methods. This model is applied to cerebral organoids, where it is shown that limitations in diffusion and organoid size can be partially overcome by localizing metabolically-active cells to an outer layer in a sphere, a regionalization process that is known to occur through neuroglial precursor migration both in organoids and in early brain development. The given prototypical solutions include a review of metabolic information for many cell types and can be broadly applied to many forms of tissue constructs. This work enables researchers to model oxygen and nutrient delivery to cells, predict cell viability, study dynamics of mass transport in 3D tissue constructs, design constructs with improved diffusion capabilities, and accurately control molecular concentrations in tissue constructs that may be used in studying models of development and disease or for conditioning cells to enhance survival after insults like ischemia or implantation into the body, thereby providing a framework for better understanding and exploring the characteristics and behaviors of engineered tissue constructs.
Reference: McMurtrey RJ. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue Engineering. doi: 10.1089/ten.TEC.2015.0375
Dr. McMurtrey may receive numerative benefit from his research.
Return to top of page
|
|
|
Jan 7, 2016 Fetal Timeline Maternal Timeline News News Archive
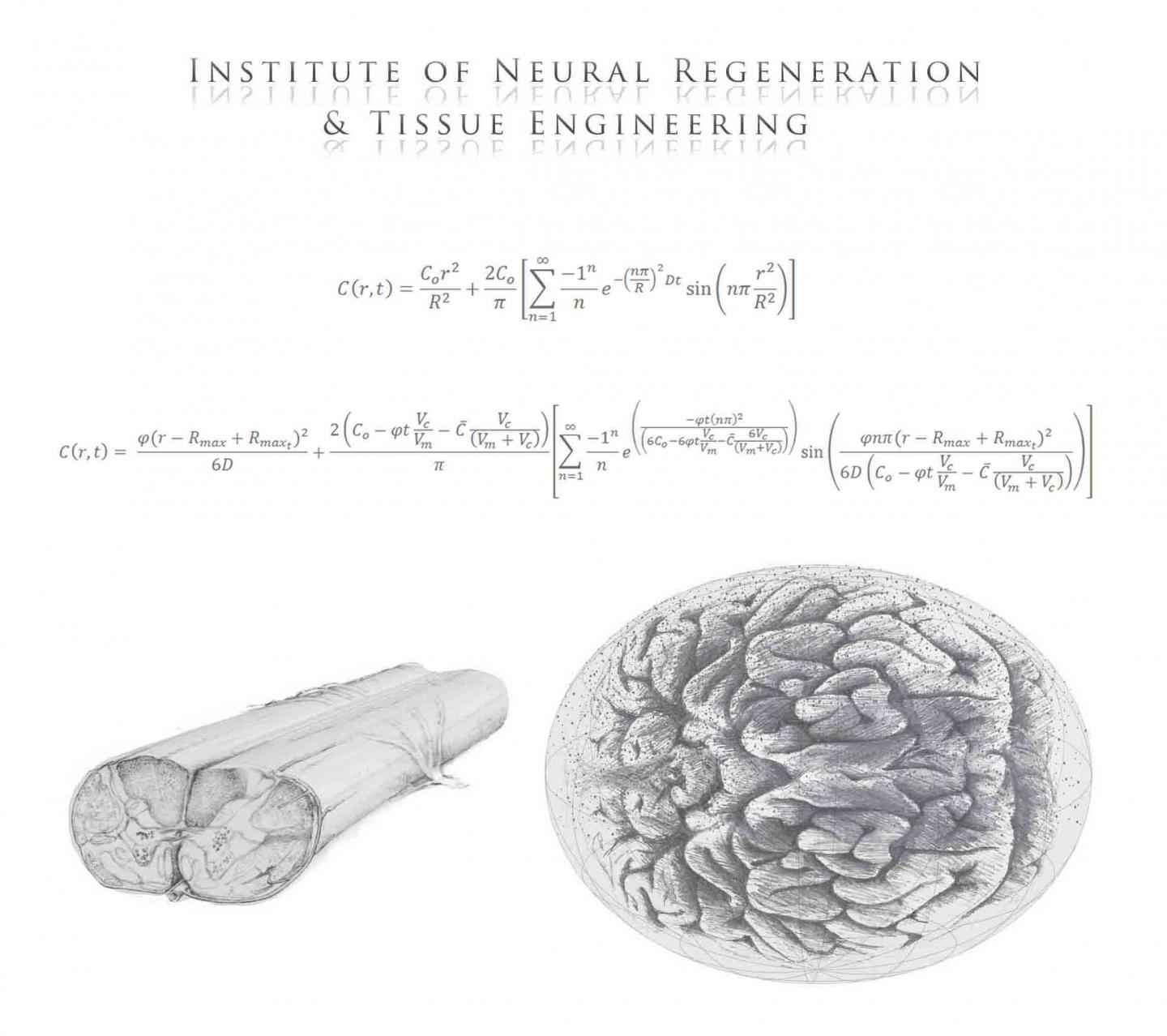
Models for 3-D neural tissue will hopefully provide new tools and nsight
into tissue development, disease modeling, and regenerative medicine.
Image Credit: Institute of Neural Regeneration & Tissue Engineering
|
|
|
|



