|
|
Slow stem cell division causes small brain?
Duke University research has followed the development of microcephaly, which produces a much smaller brain than normal, to find stem cells were simply moving too slowly when constructing neurons in affected brains.
Microcephaly is rare and causes intellectual disability and seizures. A genetic form of the disorder occurs as the fetal brain develops in pregnancy. Particularly vulnerable is the cerebral cortex, the large brain structure responsible for abstract thought, memory and language.
In 2010, Debra Silver PhD, Assistant Professor in Molecular Genetics and Microbiology at Duke University School of Medicine, and member of the Duke Institute for Brain Sciences, with her group published in Nature Neuroscience a discovery that mice missing a copy of the Magoh (mouse) gene have severely reduced brain size. A physical error reminiscent of genetic microcephaly in people. "At the time, we really didn't know why," added Silver.
The group then zeroed in on neural stem cells which divide to form either a new stem cell or the beginning of a new neuron cell.
Publishing in the journal Neuron, their new findings provide a mechanical explanation for microcephaly —that Magoh (mice) and MAGOH (human) genes control the timing of stem cell division in the brain. This information adds to our understanding of autism and other neurodevelopmental disorders where a disruption occurs in the numerical balance of developing brain neurons.
"This study shows that the time it takes for a stem cell to divide matters in brain development.
"But beyond microcephaly, I think it's going to be relevant to stem cell dysfunction changing the performace of other cells in the body."
Debra Silver PhD, Assistant Professor, Molecular Genetics and Microbiology, Duke University School of Medicine, and member of the Duke Institute for Brain Sciences.
The 2010 study found neural stem cells seemed out of balance with the number of neurons being produced in Magoh-deficient mouse brains.
Researchers suspected timing in the process of stem cell division during mitosis — or cell division that produces tissues and organs — could be cause for this imbalance. Other microcephaly-linked genes are known to control mitosis, but how defects are caused was not known.
In the new study, Silver's team found about 30% of stem cells in mice without the Magoh gene took longer — in some cases two or three times longer — to divide.
The scientists used cutting-edge, live imaging techniques to watch those cells. They were surprised to see even sluggish stem cells differentiated into neurons — but were more likely to die.
"It's really a combination that helps explain microcephaly. On one hand, you're really not making enough new stem cells, and if you don't have enough stem cells you can't make enough neurons in the brain. On the other hand, some neurons getting made then die."
Debra Silver PhD
Silver's team saw similar results (premature differentiation and death) when they extended the process of cell division, using two different drugs, in genetically normal mice. Experiments suggest both death and differentiation are distinct outcomes of delayed stem cell growth.
Magoh is a protein that switches on many important genes, but it does not work alone. In fact, previous research has linked Magoh's partners in gene regulation to other neurodevelopmental diseases.
The Duke team is now planning large-scale screening approaches to ask what other genetic pathways influence the decision moment when a slowed stem cell makes a neuron.
Silver's team continues to explore cell division in healthy brains with preliminary data supporting timing in stem cell division is critical.
Studies published more than a decade ago also show that mitotic cell division takes increasingly longer as brain development progresses.
"We now have an experimental system in place to help us understand why more neurons are made as brain development proceeds."
Debra Silver PhD
Abstract: "Prolonged Mitosis of Neural Progenitors Alters Cell Fate in the Developing Brain
Highlights
•Mitotically delayed Magoh+/− radial glia directly produce altered progeny
•Pharmacological prolonging of mitosis recapitulates Magoh+/− progenitor phenotypes
•Prolonged progenitor mitosis causes increased neurogenic and apoptotic divisions
•Apoptosis and differentiation are mutually exclusive outcomes of mitotic delay
Summary
Embryonic neocortical development depends on balanced production of progenitors and neurons. Genetic mutations disrupting progenitor mitosis frequently impair neurogenesis; however, the link between altered mitosis and cell fate remains poorly understood. Here we demonstrate that prolonged mitosis of radial glial progenitors directly alters neuronal fate specification and progeny viability. Live imaging of progenitors from a neurogenesis mutant, Magoh+/−, reveals that mitotic delay significantly correlates with preferential production of neurons instead of progenitors, as well as apoptotic progeny. Independently, two pharmacological approaches reveal a causal relationship between mitotic delay and progeny fate. As mitotic duration increases, progenitors produce substantially more apoptotic progeny or neurons. We show that apoptosis, but not differentiation, is p53 dependent, demonstrating that these are distinct outcomes of mitotic delay. Together our findings reveal that prolonged mitosis is sufficient to alter fates of radial glia progeny and define a new paradigm to understand how mitosis perturbations underlie brain size disorders such as microcephaly.
CITATION: "Prolonged Mitosis of Neural Progenitors Alters Cell Fate in the Developing Brain" Louis-Jan Pilaz, John J. McMahon, Emily E. Miller, Ashley L. Lennox, Aussie Suzuki, Edward Salmon, Debra L. Silver. Neuron, January 7, 2016. DOI: 10.1016/j.neuron.2015.12.007
The study was funded by the National Institutes of Health (R01NS083897) and the Duke Cancer Center (P30 CA014236).
Return to top of page
|
|
|
Jan 15, 2016 Fetal Timeline Maternal Timeline News News Archive
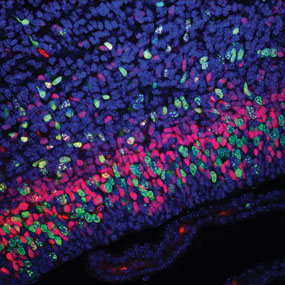
Magoh is a candidate gene responsible for brain microcephaly.
Brain structure and size requires precise division of neural stem cells (NSCs).
When stem cells self-renew, they generate intermediate neural progenitors (INPs) and neurons.
Factors that regulate NSCs and mechanically aberrant NSC division causes reduced brain size.
Magoh, a component of the exon junction complex (EJC) binds RNA, controlling mouse
cerebral cortical size by regulating NSC division. A Magoh haplo-insufficiency causes
microcephaly due to INP depletion and neuronal apoptosis.
This confocal micrograph of a mouse brain shows GFP (green) and stained,
Tbr2 (red) and DAPI (blue). The Magoh gene, is completely conserved between
mice and humans. In humans it is identified as the MAGOH gene.
Image Credit: Nature Neuroscience Reviews
|
|
|
|



