|
|
Linking fetal to adult brain disorders
Using mice as their model animal, scientists have outlined the possible mechanics behind some common neurodevelopment disorders.
Fetal development is known to affect later adult behavior, this is fundamental in nearly all organisms. Similarly autism, as a highly inheritable neurodevelopment disorder causing difficulties in social interactions, has been thought to be caused by an overgrowth of neurons during the prenatal period. But, the precise timing and cause of this overgrowth has been difficult to pinpoint.
Now, researchers at Case Western Reserve University School of Medicine and the University of California San Francisco (UCSF) School of Medicine, have uncovered abnormalities in mouse embryo brains they feel are responsible for abnormal adult mouse brain structures — with all the attending behavioral abnormalities.
Their findings demonstrate a fetal origin for social and repetitive behavior deficits, such as those seen in disorders like autism.
Researchers used engineered mice with mutations in two Disheveled genes — Dvl1 and Dvl3 — in order to identify a critical period in embryonic brain development.
The early corticogenesis period of brain development is in the deepest layer of cortical neurons. It is brought on by a temporary expansion of basal neural progenitor cells (NPCs) after deregulation of a β-catenin/Brn2/Tbr2 transcriptional cascade event. A temporary pharmacological intervention in the Wnt pathway in this early period, rescued the β-catenin/Brn2/Tbr2 transcriptional cascade.
Remarkably, this treatment prevented adult behavioral deficit and partially rescued abnormal brain structures found in Dvl mutant mice — by expanding the quantity of basal neural progenitor cells (NPCs) after deregulating the β-catenin/Brn2/Tbr2 transcriptional cascade.
After identifying these abnormalities, the scientists were able to intervene and treat the fetal mice — which developed without any behavioral deficits.
Further work is still required to determine if any abnormal development occurrs later in adult brain circuits or function. And more study is required to develope more opportunities in therapeutic intervention. This study was published in the February 2016 issue of Molecular Psychiatry.
"By defining the pathway that connects embryonic development to adult social conditions, we were able to target the same pathway in the embryo and provide a potential approach to stop development of abnormal behaviors.
"In the mice we treated, we were able to reverse the embryonic deficits that appear to lead to social and repetitive behavior disorders."
Anthony Wynshaw-Boris, MD, PhD, Chair, Department of Genetics and Genome Sciences, Case Western Reserve University School of Medicine and University Hospitals Case Medical Center.
Abstract
Social interaction is a fundamental behavior in all animal species, but the developmental timing of the social neural circuit formation and the cellular and molecular mechanisms governing its formation are poorly understood. We generated a mouse model with mutations in two Disheveled genes, Dvl1 and Dvl3, that displays adult social and repetitive behavioral abnormalities associated with transient embryonic brain enlargement during deep layer cortical neuron formation. These phenotypes were mediated by the embryonic expansion of basal neural progenitor cells (NPCs) via deregulation of a β-catenin/Brn2/Tbr2 transcriptional cascade. Transient pharmacological activation of the canonical Wnt pathway during this period of early corticogenesis rescued the β-catenin/Brn2/Tbr2 transcriptional cascade and the embryonic brain phenotypes. Remarkably, this embryonic treatment prevented adult behavioral deficits and partially rescued abnormal brain structure in Dvl mutant mice. Our findings define a mechanism that links fetal brain development and adult behavior, demonstrating a fetal origin for social and repetitive behavior deficits seen in disorders such as autism.
This research was supported by NINDS grants R01 NS073159 (AWB) and R01NS079231 (RYB & NA), the Simons Foundation SFARI #256769 (NA), the Ontario Brain Institute (JPL), and an Autism Speaks Translational Postdoctoral Fellowship #7587 (HB). Behavioral data were obtained with the help of the Gladstone Institute Behavioral Core (supported by NIH grant P30NS065780). This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
About Autism
Autism and autism spectrum disorder (ASD) refer to a group of complex neurodevelopment disorders characterized by repetitive and characteristic patterns of behavior and difficulties with social communication and interaction. The U.S. Centers for Disease Control and Prevention (CDC) estimates that about 1 in 68 children has been diagnosed with ASD.
About Case Western Reserve University School of Medicine
Founded in 1843, Case Western Reserve University School of Medicine is the largest medical research institution in Ohio and is among the nation's top medical schools for research funding from the National Institutes of Health. The School of Medicine is recognized throughout the international medical community for outstanding achievements in teaching. The School's innovative and pioneering Western Reserve2 curriculum interweaves four themes--research and scholarship, clinical mastery, leadership, and civic professionalism--to prepare students for the practice of evidence-based medicine in the rapidly changing health care environment of the 21st century. Nine Nobel Laureates have been affiliated with the School of Medicine. Annually, the School of Medicine trains more than 800 MD and MD/PhD students and ranks in the top 25 among U.S. research-oriented medical schools as designated by U.S. News & World Report's "Guide to Graduate Education." The School of Medicine's primary affiliate is University Hospitals Case Medical Center and is additionally affiliated with MetroHealth Medical Center, the Louis Stokes Cleveland Department of Veterans Affairs Medical Center, and the Cleveland Clinic, with which it established the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University in 2002.
http://www.case.edu/medicine.
Return to top of page
|
|
|
Feb 11, 2016 Fetal Timeline Maternal Timeline News News Archive
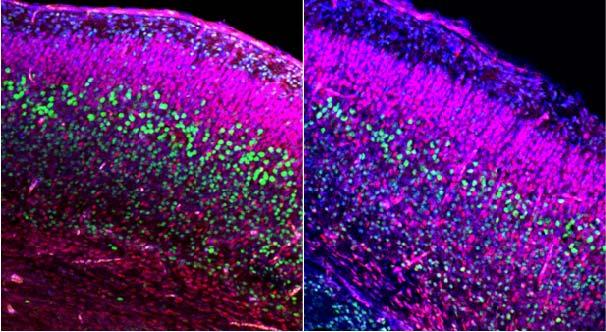
Control (left) and mutant (right) mouse embryonic brains at day 18.5 (birth typically at day 20). Both are
stained to highlight all cell nuclei (Blue), and deep layer neurons and anti-Cux1 (Red).
Analysis shows specific reduction in deep layer (Ctip2+) neurons at day 18.5.
This reduction was preceded by brain over-growth at day 14.5.
Image Credit: Case Western Reserve School of Medicine
|
|
|
|



