|
|||||||||||||||
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
|||||||||||||||||||||||||||
Potential to treat hypertrophic cardiomyopathy More than 15 years ago, researchers discovered the precise malfunction of a specific protein in the heart that leads to hypertrophic cardiomyopathy (HCM), a common culprit in the sudden death of young athletes. Now, a team of scientists led by David Warshaw, has used their earlier findings to develop a possible treatment to prevent HCM, an inherited disease which can cause the heart to thicken and stop effectively pumping blood — ending in heart failure. Warshaw is professor and chair of molecular physiology and biophysics at the University of Vermont (UVM) College of Medicine. He wrote about the significance of this potential therapy for a "Perspectives" column in the February 5, 2016 issue of the journal Science. "This may offer a generalized approach to solving hypertrophic cardiomyopathy," says David Warshaw PhD, who is also an investigator in the Cardiovascular Research Institute of Vermont at UVM. "I think it's extremely promising."
A mutation of myosin can "alter the motor's power-generating capacity" and make the heart work improperly, which in turn causes the heart to enlarge, Warshaw says. For many years, scientists assumed that the mutation caused the myosin to lose its motoring power, throwing off the whole heart engine. But in a study Warshaw published in 2000 in Circulation Research, he and colleagues found that the problem wasn't diminished power in the myosin, it was too much power in myosin with this mutation. "By analogy, placing the engine of an Indy race car (i.e., mutant myosin) in a stock car chassis (i.e., the heart's connective tissue matrix) could lead to internal stress and structural damage," Warshaw writes in his "Perspectives" article. "For the heart, this amounts to inducing cardiac fibrosis and muscle cell disarray characteristic of HCM patients." The team of scientists who found a way to address this problem - which they report in the February 5, 2016 issue of Science - are from Harvard Medical School, Stanford University School of Medicine, University of Colorado, and MyoKardia Inc. in San Francisco, a biotechnology company formed to develop such treatments. Using mice bred with the mutation, the team tested a molecular inhibitor that dials down the power of the myosin motor to a more normal level. As early as eight weeks old, mice in the study that received the drug containing the molecule did amazingly well and the HCM was prevented from surfacing. "When they gave the drug to a young mouse with the mutation, the mouse's heart developed normally," Warshaw adds.
Nonetheless, Warshaw sees great potential. In previous studies, he found mutations to other heart proteins also increased the heart muscle, leading to HCM. Warshaw believes the same molecule could be used on the myosin motor to compensate and block HCM in all related cases. Abstract: Science Perspectives: "A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice" Summary: Science Magazine: "Throttling back the heart's molecular motor" |
Feb 17, 2016 Fetal Timeline Maternal Timeline News News Archive 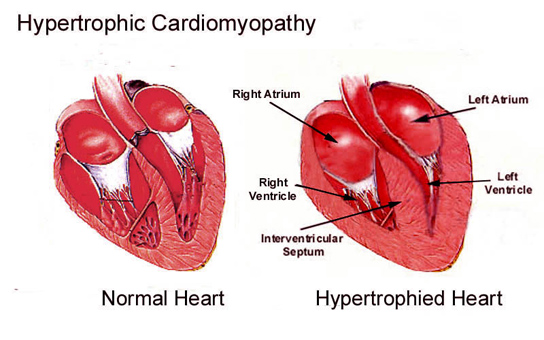
Scientists have identified a small molecule, MYK-461, that reduces contractility of the heart muscle
|
|||||||||||||||||||||||||||

