|
|
Copper key to brain cell development
Chemical change spurs the rapid transport of copper.
Researchers at Johns Hopkins used a precision sensor in a chicken embryo and found dramatic differences between the use of copper in developing and fully mature neurons.
Investigators say their findings reveal how brain cells quickly adjust copper use from energy production and defense against free radicals, to activating enzymes that make neurons into neurons.
Yuta Hatori PhD, is a postdoctoral fellow in the laboratory of Svetlana Lutsenko PhD, professor of physiology at the Johns Hopkins University School of Medicine. Hatori used a protein sensor that changes its fluorescence to signal the so-called redox state of cells, which refers to the ability of molecules to exchange electrons, driving many cellular processes.
Working with colleagues in the school of medicine's Department of Neuroscience, the team infected chicken embryos at different stages of development with a gene encoding a tiny sensor, allowing them to observe the redox state of neurons as they mature.
"Biochemical studies have revealed many proteins are involved in neural differentiation — which requires copper. We knew there was a big spike in the brain's copper levels at a certain stage of development. With our new results, we now know a lot more about how developing neurons use copper for their various needs."
Svetlana Lutsenko PhD, Professor of Physiology, the Johns Hopkins University School of Medicine
Cells control their internal redox states by precisely tuning the ratio of two small molecules: glutathione and glutathione disulfide.
Working with colleagues in the school of medicine's Department of Neuroscience led by Shanthini Sockanathan, the team infected chicken embryos at different stages of development with a gene encoding a tiny sensor. They found that the redox state of neurons control movement changes as they mature. Their report is published in Nature Communications.
Delving deeper, Lutsenko's team found one effect of the changed redox state is an exposed copper-binding site on the Atox1 protein, known to shuttle copper around the inside of a cell.
Cells that are differentiating turn out to make more Atox1 along with a related protein, ATP7A. Working together, both proteins direct copper into a "secretory pathway," increasing the amount of copper to a copper-requiring enzyme responsible for signals between neurons.
The importance of getting copper to the right place in the cell at the right time may shed light on processes beyond development.
For example, aging is known to wear on a cells' precise control of it's redox state. "Our study suggests that small redox changes may have big effects on proteins in the secretory pathway, which are very important to brain function," Lutsenko adds.
Armed with a better understanding of how normal neurons use copper, Lutsenko's team plans to look next at what happens when that process goes wrong, as in a copper-processing disorder known as Wilson's disease.
Abstract
Brain development requires a fine-tuned copper homoeostasis. Copper deficiency or excess results in severe neuro-pathologies. We demonstrate that upon neuronal differentiation, cellular demand for copper increases, especially within the secretory pathway. Copper flow to this compartment is facilitated through transcriptional and metabolic regulation. Quantitative real-time imaging revealed a gradual change in the oxidation state of cytosolic glutathione upon neuronal differentiation. Transition from a broad range of redox states to a uniformly reducing cytosol facilitates reduction of the copper chaperone Atox1, liberating its metal-binding site. Concomitantly, expression of Atox1 and its partner, a copper transporter ATP7A, is upregulated. These events produce a higher flux of copper through the secretory pathway that balances copper in the cytosol and increases supply of the cofactor to copper-dependent enzymes, expression of which is elevated in differentiated neurons. Direct link between glutathione oxidation and copper compartmentalization allows for rapid metabolic adjustments essential for normal neuronal function.
Other authors on the paper are Ye Yan, Katharina Schmidt, Eri Furukawa, Nesrin M. Hasan, Nan Yang and Chin-Nung Liu, all of the Johns Hopkins University School of Medicine.
The study was funded by the National Institute of General Medical Sciences (grant number R01 GM101502), the National Institute of Diabetes and Digestive and Kidney Diseases (grant number DK071865), and the National Institute of Neurological Disorders and Stroke (grant number NS046336).
Return to top of page
|
|
|
Feb 18, 2016 Fetal Timeline Maternal Timeline News News Archive
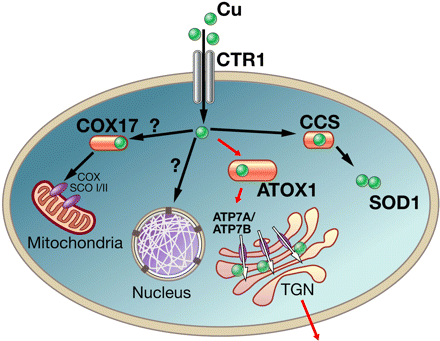
Copper enters the cell through copper transporter Ctr1.
Cox17 together with Sco proteins facilitates incorporation of copper into cytochrome-c oxidase (COX).
CCS transfers copper to cytosolic SOD1. Red arrows indicate the pathway. In this pathway,
Cu-ATPases receive copper from ATOX1, transferring copper into the secretory pathway,
as well as exporting excess copper from the cell.
Image Credit: Svetlana Lutsenko, Physiological Reviews Jul 2007
|
|
|
|



