|
|
How the nervous system trims its branches
As tiny embryos, we start out with a lot more neuronal material than we actually need. While developing, our body drastically prunes excess by cutting branches called axons from nerves — and sometimes pruning entire neurons.
Scientists have assumed for a long time that the decision to cut or keep an axon was coordinated by the axon itself — not by the cell body from which it extends. In recent years, clues from several research groups challenged that assumption. Now research at Rockefeller University has proven that the cell body does actively control axon degeneration, while uncovering many molecular details explaining this long-range communication.
Published February 18 in the journal Cell, scientists reveal that instructions from the cell decide whether an axon lives or dies.
"This is a surprising finding. When the axon senses a 'distress signal' — a lack of neural growth factor (NGF) — it doesn't make the decision to degenerate by itself. It actually sends the information to the cell body, which is quite far away, and the cell body sends its decision back down to the axon."
Marc Tessier-Lavigne PhD, President of Rockefeller University, Carson Family Professor in the Laboratory of Brain Development and Repair, and senior author.
The survival of some axons depends on the presence of neural growth factor or NGF in their local environment, a sign of plenty of nutrients being around. "Because NGF is present at the tips of axons, far from cell body, it has been hard for scientists to identify that the cell body played a central role in axon degeneration," according to co-first author David Simon, a postdoctoral fellow in the lab of Tessier-Lavigne. "And indeed, our study confirmed that all the cellular machinery that is needed to die is present in the axon. However the axon needs a message from the cell body to know when to degenerate."
Tessier-Lavigne, and Simon, and co-first author Jason Pitts, and their colleagues severed the connection between axons and the cell body in mouse neurons, and then removed NGF. This triggered distress signals that informed the neurons that an axon should degenerate. However, they also observed that only axons still connected to their cell bodies actually did degenerate.
"When axons have been isolated from their cell body, the process that normally leads to degeneration does not turn on," concludes Simon.
Researchers further observed that when NGF levels drop, the cell body activates a gene that makes a protein called Puma that triggers the axon to die.
The idea that the cell body coordinates the degeneration of axons may be new to neuroscientists, but it does make biological sense.
While an axon only detects signals within its immediate environment, the cell body receives many signals regarding growth. For example, sensory neurons convey pain from the surface of the skin, where NGF levels fluctuate. Simon: "Our findings show the axon won't make the decision to die simply based on an immediate drop in NGF."
The team proposes that by leaving the decision up to the cell body, the neuron has a "fail safe" that prevents unnecessary degeneration.
Potential implications apply to excessive axons degeneration. In some neurodegenerative diseases, affected neurons adapt to pathways found during normal embryo development which lead to axon pruning. This suggests diseases may mistakenly promote axon degeneration due to errors in cell body signalling.
"If researchers could find a way to interfere with the expression or function of Puma, that could be very neuroprotective, and potentially protect neurons from degenerating."
David Simon PhD, postdoctoral fellow, Tessier-Lavigne laboratory, and co-first author.
Abstract Highlights
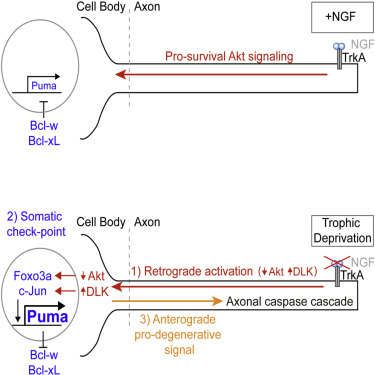
•The cell body is an active and essential regulator of distal axon degeneration
•The cell body integrates retrograde loss of Akt and gain of JNK signaling
•Pro-apoptotic Puma in the cell body initiates an anterograde degenerative program
Summary
During development, sensory axons compete for limiting neurotrophic support, and local neurotrophin insufficiency triggers caspase-dependent axon degeneration. The signaling driving axon degeneration upon local deprivation is proposed to reside within axons. Our results instead support a model in which, despite the apoptotic machinery being present in axons, the cell body is an active participant in gating axonal caspase activation and axon degeneration. Loss of trophic support in axons initiates retrograde activation of a somatic pro-apoptotic pathway, which, in turn, is required for distal axon degeneration via an anterograde pro-degenerative factor. At a molecular level, the cell body is the convergence point of two signaling pathways whose integrated action drives upregulation of pro-apoptotic Puma, which, unexpectedly, is confined to the cell body. Puma then overcomes inhibition by pro-survival Bcl-xL and Bcl-w and initiates the anterograde pro-degenerative program, highlighting the role of the cell body as an arbiter of large-scale axon removal.
Return to top of page
|
|
|
Feb 25, 2016 Fetal Timeline Maternal Timeline News News Archive
.jpg)
[TOP] Normal axons on neurons, degenerate [white dot patterns] when deprived of NGF.
[BOTTOM] However, axons on neurons without the Puma protein remain intact.
Image Credit: Laboratory of Brain Development and Repair, The Rockefeller University/Cell
|
|
|
|




.jpg)