|
|||||||||||||||
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
|||||||||||||||||||||||||||
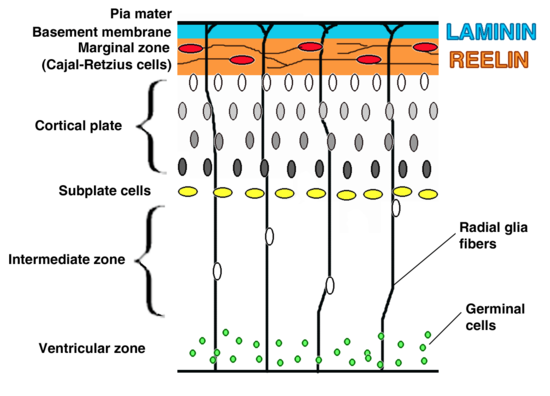
Mom's bacterial infection can change fetal brain The study appears today in the journal Cell Host & Microbe.
"The finding was unexpected, pneumococcal infections in children and adults can lead to meningitis and death of neurons," said the study's corresponding author Elaine Tuomanen, MD, and chair of the St. Jude Department of Infectious Diseases. "This study in a mouse model of bacterial infection found that prenatally the opposite is true. Evidence suggests maternal infections cause a signaling event that leads to proliferation and reorganization of neurons in a developing brain, which becomes defective in some way — maybe due to overcrowding." Researchers revealed for the first time that pieces of the bacterial cell wall cross through the maternal placenta and travel into the fetal brain. This triggers proliferation of immature neurons. Evidence suggests the proliferation of neurons is sparked by a previously unrecognized pathway involving the immune system and a protein which regulates gene expression (or whether a gene is turned on or not turned on).
Investigators saw that mice exposed to pieces of bacterial cell wall early in fetal development, performed below average on tests measuring memory and cognitive function in mice after birth. They also found evidence that treatment of moms using the antibiotic ampicillin, which destroys bacteria and sends pieces of the bacterial cell wall into the bloodstream, still led to neuronal increase. Tuomanen said the results raise questions about which class of antibiotics should be used to treat bacterial infections in pregnancy. "This study suggests widely used antibiotics like ampicillin — that cause bacteria to burst cell walls — may lead to changes in the developing brain," she said. "Such changes did not occur in mice treated with antibiotics like clindamycin, which kills without releasing cell wall material. "Additional studies are needed to understand the long-term impact of different classes of antibiotics on pregnancy outcomes," Tuomanen said.
Within hours of being in the fetal brain, instead of triggering inflammation and cell death, bacterial cells switched on the protein FoxG1. FoxG1 drives neural proliferation.
"Additional research is needed to understand how bacterial cell wall induces proliferation via this newly identified path linking the innate immune receptor TLR2 with the transcription factor FoxG1 — to drive neural proliferation in the fetus. This mechanism might lead to new strategies to repair or replacement of neurons lost to illness and injury," adds Dr. Tuomane . Abstract Highlights Beth Mann, of St. Jude, and Jessica Humann, Ph.D., formerly of St. Jude and now of Florida Agricultural and Mechanical University, Tallahassee, Fla., are the first authors. The other authors are Geli Gao, Philip Moresco, Joseph Ramahi, Lip Nam Loh, Arden Farr and Richard Smeyne, all of St. Jude; and Yunming Hu, Kelly Durick-Eder and Sophie Fillon, all formerly of St. Jude. The research was funded in part by grants (CA02176535, CA23944, R0127913) from the National Institutes of Health, and ALSAC. |
Mar 15, 2016 Fetal Timeline Maternal Timeline News News Archive
|
|||||||||||||||||||||||||||