|
|
Can we turn off Alzheimer's disease?
Russian scientists have found a 'trigger' to Alzheimer's. By figuring out the steps involved in it's development, they may possibly have determined the trigger event beginning the conversion of normal proteins into the plaques of Alzheimer's.
Lomonosov Moscow State University (MSU) scientists, along with colleagues from the Institute of Molecular Biology, the Russian Academy of Sciences and King's College London, have succeeded in sorting out the mechanisms involved in Alzheimer's development.
Their work is published in the journal Nature: Scientific Reports.
"Alzheimer's disease is seen as widespread degenerative damage of the central nervous system, leading to a loss of mental ability. Until now it was considered incurable," explains Vladimir Polshakov PhD, MSU Faculty of Fundamental Medicine, and research leader.
Several hypotheses have been generated to explain its development. One of the most common is the amyloid hypothesis. Amyloids (more precisely, beta-amyloid peptides) are protein molecules that normally protect brain cells. After fulfilling their function, used up beta amyloid peptides are cut up by proteases to become harmless 'slag' to be reclaimed for use, or be discarded from the body.
However, according to this 'beta-amyloid hypothesis' — something goes wrong and these protector cells are turned into killer cells. Beta-amyloid peptides begin to form cell aggregates too large for proteases' to cut up and dispose. When the brain becomes covered with amyloid plaques, it is considered to be in the last stages of Alzheimer's disease. However, the earlier period of beta-amyloid transformation into harmful plaques — has until now been highly unexplored.
"We know ions of several metals are crucial to the initiation of plaques. Primary is zinc. Zinc actually conducts a number of useful and healthy functions in the brain. Though in this case, it was suspected to be a 'pest' and initiator of processes leading to Alzheimer's.
"However, what was not clear was exactly what happens between zinc and peptide molecules. Which amino acids bind zinc? And how does this initiate peptide aggregation? Our goal became to throw light on these questions".
Vladimir Polshakov PhD, Faculty of Fundamental Medicine, Lomonosov Moscow State University, and research leader.
The team began to study various short peptide regions that bind to metal ions. A number of experimental techniques were applied, including nuclear magnetic resonance (NMR) spectroscopy, in order to visualize cellular molecules.
According to Polshakov, the choice of the pathogens they studied was 'partly luck.' One specimen was a so-called 'English mutation' — where one amino acid substitution in a peptide makes it different from the more common beta-amyloid peptides. Using NMR, scientists were able to watch the chemical and structural changes occurring when peptides interacte with zinc to create benign cell aggregates.
The 'English mutation' of a beta-amyloid peptide into a pathenogenic beta-amyloid peptide, happens from a single change in one of its amino acid atomic structures. The process, known as "isomerism", occurred spontaneously without any help from any enzymes. Isomerisation is related to the ageing of a cell — a known condition in Alzheimer's as the disease is primarily found in people over 50.
Biologists from Moscow's Institute of Molecular Biology saw that adding an isomerized peptide into mouse brains accelerated formation of amyloid plaques. Zinc ions, a metal binding domain on isomerized peptides, aggregated peptides so quickly that plaque formation was hard to follow. However, they ascertained that isomerization of peptides in the presence of zinc is at fault for creating Alzheimer's.
The trigger is the same, an aggregate seed formed by peptide dimers — two peptide molecules, connected with the help of a zinc ion.
Dimers were also detected in normal human peptides. However, the difference is in the speed of formation — and that they are prone to aggregate more densely in Alzheimer's.
Researchers therefore propose that a zinc-controlled mechanism transforms a peptide-protector into a peptide-killer.
The zinc mechanism explains multiple experimental data results, not only gathered by this group, but also by other laboratories studying Alzheimer's disease. Researchers hope the discovery will lead to new medicines which can block peptide aggregations coupled to zinc ions.
Abstract
Conformational changes of Aβ peptide result in its transformation from native monomeric state to the toxic soluble dimers, oligomers and insoluble aggregates that are hallmarks of Alzheimer’s disease (AD). Interactions of zinc ions with Aβ are mediated by the N-terminal Aβ1–16 domain and appear to play a key role in AD progression. There is a range of results indicating that these interactions trigger the Aβ plaque formation. We have determined structure and functional characteristics of the metal binding domains derived from several Aβ variants and found that their zinc-induced oligomerization is governed by conformational changes in the minimal zinc binding site 6HDSGYEVHH14. The residue H6 and segment 11EVHH14, which are part of this site are crucial for formation of the two zinc-mediated interaction interfaces in Aβ. These structural determinants can be considered as promising targets for rational design of the AD-modifying drugs aimed at blocking pathological Aβ aggregation.
Return to top of page
|
|
|
Mar 21, 2016 Fetal Timeline Maternal Timeline News News Archive
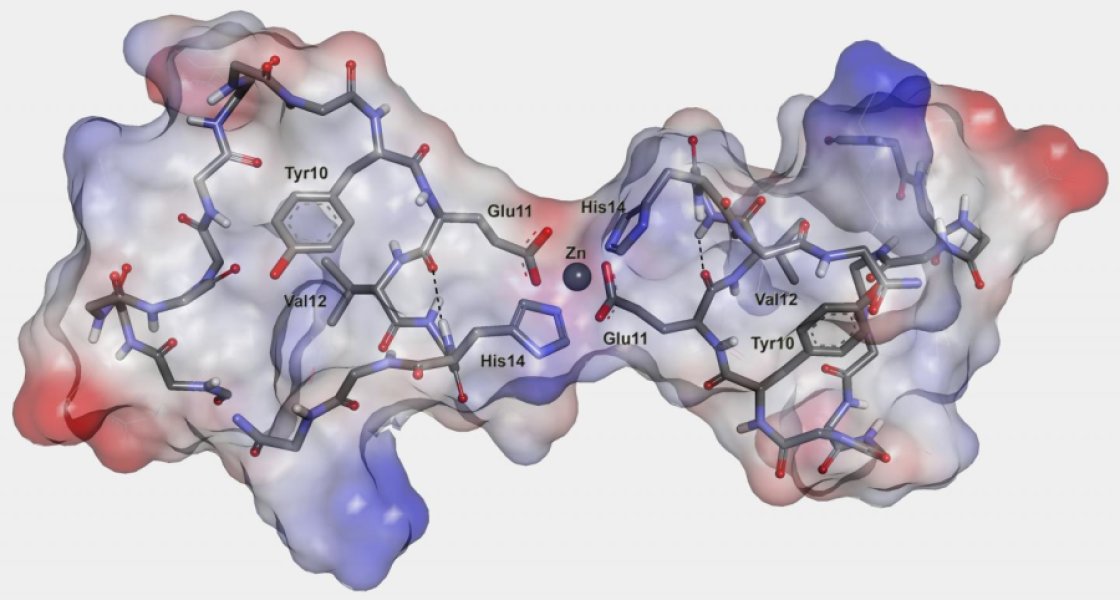
This three-dimensional illustration is of the dimer of the metal-binding domain of beta-amyloid peptide.
This is the 'English mutation' - or two peptide molecules connected to each other with a zinc ion.
Image Credit:
Lomonosov Moscow State University
|
|
|
|



