|
|
Abnormalities in early embryos may self correct
Abnormal cells in the early embryo are not necessarily a sign the baby will be born with a birth defect, suggests new research in mice from the University of Cambridge.
Researchers in the Department of Physiology, Development and Neuroscience at Cambridge University, UK, report a mouse model of aneuploidy — where some cells in an embryo contain abnormal numbers of chromosomes — are eliminated and replaced by healthy cells, repairing and in many cases completely fixing — a formerly "abnormal" embryo.
The study is published in the journal Nature Communications.
Normally, each cell in the human embryo contains 23 pairs of chromosomes — made up of 22 pairs of chromosomes and one pair of sex chromosomes.
But, some carry multiple copies of chromosomes. This can lead to developmental disorders. Children born with three copies of chromosome 21 will develop Down's syndrome.
Pregnant mothers — particularly older mothers at greatest risk of developing aneuploid disorders — are offered tests to predict the chance of genetic abnormalities. Between the 11th and 14th weeks of pregnancy, mothers may be offered chorionic villus sampling (CVS), a test involving removal and analysis of placental cells. Or a later test — amniocentesis — to analyse cells shed by the fetus into surrounding amniotic fluid. Though more accurate, amniocentesis is usually carried out after 15-20 weeks of pregnancy, when a fetus is further developed.
Professor Magdalena Zernicka-Goetz, the study's senior author, was inspired to carry out this research following her own experience when pregnant with her second child.
"I am one of the growing number of women having children over the age of 40 - I was pregnant with my second child when I was 44."
Magdalena Zernicka-Goetz PhD, Professor Mammalian Development and Stem Cell Biology, Department of Physiology, Development and Neuroscience, Gurdon Institute, University of Cambridge, Wellcome Trust/Cancer Research Institute, Cambridge, UK
At the time, a CVS test found that as many as a quarter of the cells in the placenta that joined her and her developing baby were abnormal. Could the developing baby also have abnormal cells? When Professor Zernicka-Goetz spoke to geneticists about the potential implications, she found very little was understood about the fate of embryos containing abnormal cells or anything about abnormal cells in embryos.
Fortunately for Professor Zernicka-Goetz, her son, Simon, was born healthy. "I know how lucky I was and how happy I felt when Simon was born healthy," she says.
Professor Zernicka-Goetz: "Many expectant mothers have to make a difficult choice about their pregnancy based on a test whose results we don't fully understand. But what does it mean if a quarter of the cells from the placenta carry a genetic abnormality? How likely is it the child will have cells with this abnormality, too? This is the question we wanted to answer.
"Given that the average age at which women have their children is rising, this is a question that will become increasingly important."
"In fact, abnormal cells with numerical and/or structural anomalies of chromosomes are observed in as many as 80-90% of human early stage embryos following in vitro fertilization. CSV tests may expose some of these abnormalities."
Professor Thierry Voet, Wellcome Trust Sanger Institute and the University of Leuven, Belgium, and co-senior author of the paper.
Professor Zernicka-Goetz and colleagues developed a mouse model of aneuploidy by mixing 8-cell stage mouse embryos — with normal cells — in with embryos having abnormal cells. The team used a molecule known as reversine to induce aneuploidy (abnormal chromosome number) to create the abnormal embryos.
When the mix of normal and abnormal cells was half and half, they observed abnormal cells being killed off through 'apoptosis', or programmed-cell death. This allowed normal cells to take over, resulting in an embryo where all cells were healthy.
When the mix of cells was three abnormal to one normal cell, though some abnormal cells continued to survive, the ratio of normal cells increased.
"The embryo has an amazing ability to self correct.
"We found that even when half of the cells in the early stage embryo are abnormal, the embryo can fully repair itself. If this is the case in humans too, it will mean even when early indications suggest a child might have a birth defect because there are some abnormal cells in its embryonic body — this isn't necessarily the case."
Professor Zernicka-Goetz.
Researchers will now try to determine the exact proportion of healthy cells needed to completely repair an embryo, and the precise mechanism by which abnormal cells are identified and eliminated.
Abstract
Most human pre-implantation embryos are mosaics of euploid and aneuploid cells. To determine the fate of aneuploid cells and the developmental potential of mosaic embryos, here we generate a mouse model of chromosome mosaicism. By treating embryos with a spindle assembly checkpoint inhibitor during the four- to eight-cell division, we efficiently generate aneuploid cells, resulting in embryo death during peri-implantation development. Live-embryo imaging and single-cell tracking in chimeric embryos, containing aneuploid and euploid cells, reveal that the fate of aneuploid cells depends on lineage: aneuploid cells in the fetal lineage are eliminated by apoptosis, whereas those in the placental lineage show severe proliferative defects. Overall, the proportion of aneuploid cells is progressively depleted from the blastocyst stage onwards. Finally, we show that mosaic embryos have full developmental potential, provided they contain sufficient euploid cells, a finding of significance for the assessment of embryo vitality in the clinic.
Reference Article
Bolton, H et al. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nature Comms; 26 March 2016
Return to top of page
|
|
|
Apr 7, 2016 Fetal Timeline Maternal Timeline News News Archive
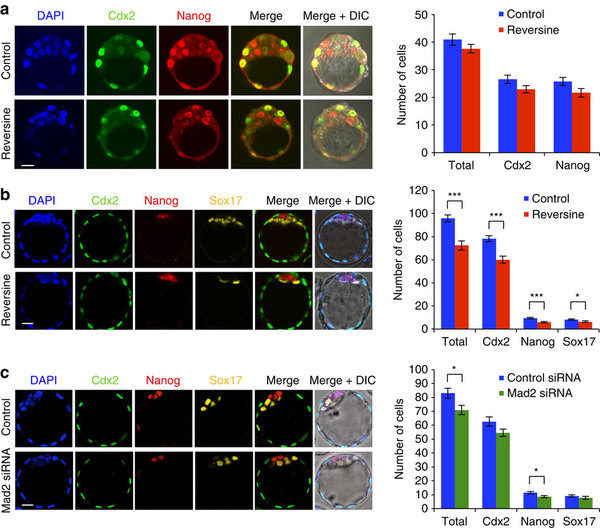
(a) Embryos treated with reversine and analysed for gene lineage in EARLY blastocyst stage, compared
to controls. No difference in number of Cdx2-positive and Nanog-positive cells observed.
(b) Embryos treated with reversine and analysed for lineage in EXPANDED blastocyst stage, compared to controls. Reversine-treated embryos show significant reduced cell number in all lines,
but segregation of cell lines not affected.
(c) Embryos injected with Mad2 siRNA or control siRNA, analysed at EXPANDED blastocyst stage.
Mad2 siRNA-injected embryos had significantly fewer cells than control siRNA-injected embryos,
but this effect was not as clear as seen with reversine treatment.
Early Blastocyst
_______________________________________
Cdx2 protein is involved in cell growth and differentiation. This protein
also plays a major
role in early embryonic development of the intestinal tract.
Sox genes are involved in sex determination. There are 20 SOX genes present in humans and mice.
NANOG is a is a protein that binds to specific DNA sequences, controlling the rate of
transcription of genetic information from DNA to messenger RNA.
In embryonic stem cells (ESCs), it is
key in cell pluripotency.
Image Credit: Zernicka-Goetz Laboratory, Gurdon Institute, University of Cambridge, UK
|
|
|
|