|
|
How early female human embryo controls X
There are considerable differences in embryo development between humans and mice, the most commonly used animal model. Research reveals human X chromosome genes are regulated differently.
Researchers at Karolinska Institutet and the Ludwig Cancer Research in Stockholm, Sweden have conducted a detailed analysis of embryo molecules in their first week of development. The work is published in the journal Cell.
Early human embryo development is difficult to study, most of our knowledge comes from mice. During the first seven days of fertilization, an egg develops from a single cell to a blastocyst, a hollow cluster of 200 to 300 cells. It is during this time the first three cell types appear: (1) trophectoderm: which gives rise to the placenta, (2) hypoblast: which forms the embryonic endoderm, and (3) enbryonic cells: which make up the embryo itself.
If the embryo is to adhere to the uterine wall and pregnancy continue, all three cell types must mature properly. However, exactly when, which order and how cell types form into humans has not been known.
By detecting gene expression in individual cells, from donated human embryos not used for IVF treatment, two research groups led by Rickard Sandberg and Fredrik Lanner have identified which genes are used in the embryo's cells at different times during the first week of development. They found that these first three cell types form later and mature more simultaneously in the human than in the mouse.
"The knowledge generated by our research not only helps us understand embryonic development better, it tells us more about how pluripotent cells are formed and regulated in early stages. This is important for use of embryonic stem cells in regenerative medicine."
Fredrik Lanner PhD, Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Sweden
Researchers also found expression of genes located on the X chromosome subject to an unexpected pattern.
Each cell in a woman has two XX chromosomes, while each cell in a man has only one, XY. To avoid women having twice the level of expression of all genes on the X chromosome as men, women's cells turn off both X genes.
In mice this process is well-studied. One of the two X chromosomes is simply shut off during the first week of development. However, it was uncertain how and even if this process begins in the first week of human embryo development.
"We've been able to demonstrate that dose balance [of X] is gradually attained days 4 to 7, interestingly through a completely new manner in which gene expression from both X chromosomes in the [human] female embryo is suppressed."
Rickard Sandberg PhD, Karolinska Institute, Department of Cell and Molecular Biology and the Ludwig Cancer Research.
Abstract Conclusion
The issue of unequal sex-chromosome dose has both emerged and been resolved many times during evolution, using diverse strategies (Deng et al., 2014b, Mank, 2009). Even between mammalian taxa, there exists separate solutions to dosage compensation (Escamilla-Del-Arenal et al., 2011), and XIST is an exclusively eutherian invention. Intriguingly, the conventional XCI model where one of the two X chromosomes is inactivated, as demonstrated in the mouse (Mak et al., 2004, Okamoto et al., 2005), does not satisfactorily explain the dynamics of X chromosome expression we observed in human preimplantation development. Instead, the data fit better with a model of an initially dual and partial expression dampening of the two X chromosomes. XIST represents an obvious candidate as a mediator for this dampening. However, the possibility that another system, conceivably the evolutionary traces of a more ancient dosage compensation mechanism, might act as a second layer of compensation in human preimplantation development should also be considered.
Finally, the transcriptional atlas of the human preimplantation embryo we provide here has unprecedented cellular and temporal resolution and will therefore be a unique resource in future research aiming to better understand human development and embryonic stem cells.
Lead authors of this study are: Sophie Petropoulos, Daniel Edsgärd and Björn Reinius.
Sophie Petropoulos has a scholarship from the Mats Sundin Fellowship Programme for an exchange between Karolinska Institutet and the University of Toronto. Other funding bodies have been the Swedish Research Council, the Ragnar Söderberg Foundation, the Swedish Foundation for Strategic Research, the European Research Council, the Åke Wiberg Foundation, and the Ludwig Cancer Research.
Publication: 'Single-cell RNA-seq reveals lineage and X chromosome dynamics in human preimplantation embryos', Sophie Petropoulos, Daniel Edsga?rd, Bjo?rn Reinius, Qiaolin Deng, Sarita Pauliina Panula, Simone Codeluppi, Alvaro Plaza Reyes, Sten Linnarsson, Rickard Sandberg, and Fredrik Lanner, Cell, published online 7 April 2016, doi: 10.1016/j.cell.2016.03.023.
About Ludwig Cancer Research: http://www.ludwigcancerresearch.org
About Karolinska Institutet - a medical university: ki.se/english
Return to top of page
|
|
|
Apr 8, 2016 Fetal Timeline Maternal Timeline News News Archive
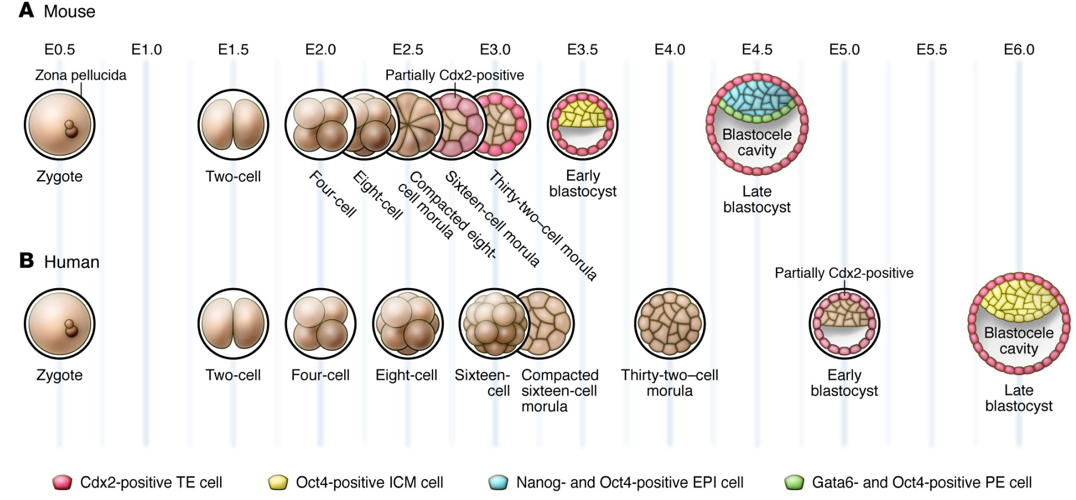
Image Credit: Katie Cockburn, Janet Rossant; Journal of Clinical Investigation 2010
|
|
|
|



