|
|
Individuals respond differently to Zika?
One of Zika's mysteries is how the virus passes from an infected mother, through the placenta, to a developing fetus. The route may not be direct either — transmission via multiple cell types may be necessary as it is transmitted through several routes, including mosquito bites, sexual contact, and blood transfusion.
Researchers studying a small sample of five donated full-term human placentas, have identified cell types that might be more vulnerable to the Zika virus strain currently circulating in the Americas. The five placentas also showed individual variability in the amount of viral spread, inflammation, and antiviral genes expressed. This likely reflects differences in individual genetic response to the infection.
"A concept that is emerging is how the host's genetics or other non-viral factors — including nutrition and her own microbiota — influence her immune response."
Mehul S. Suthar PhD, Department of Pediatrics, Division of Infectious Diseases, Emory University School of Medicine, and Emory Vaccine Center, Yerkes National Primate Research Center, Atlanta, Georgia, USA
Researchers detected infection in Hofbauer cells, which are placental macrophage cells that originated from fetal connective tissue stem cells. They also detected infection, to a lesser extent, in cytotrophoblast cells found in the middle layer of the placenta.
"One group has recently discovered viral antigen in Hofbauer cells collected from placental tissue of a fetus that unfortunately died as a result of Zika virus infection," explains Mehul Suthar PhD, senior author and Assistant Professor in Pediatrics at the Emory University School of Medicine in Atlanta, Georgia.
"Our study indicates that this cell type may be a target for Zika virus in the placenta and replication in these cells may allow the virus to cross the placental barrier to enter fetal circulation," adds co-author Rana Chakraborty PhD, a pediatric infectious disease specialist at the Emory Vaccine Center at Yerkes National Primate Research Center in Atlanta.
The study appeared May 27, 2016 in Cell Host & Microbe.
One explanation for how the virus crosses the placental barrier is by initial infection of syntiotrophoblasts, the outermost layer of cells that surround and nurture the fetus. However, earlier work has also shown these cells can resist the virus.
The work from the Suthar Lab shows that less-differentiated cytotrophoblast cells are more vulnerable to Zika infection. Although Hofbauer cells were identified over a century ago, very little is known about them except that they are are of mesenchymal origin and are particularly numerous in early pregnancy. Mesenchymal cells are loosely associated cells that lack polarity, and are able to develop into tissues of the lymphatic and circulatory systems as they migrate easily.
Overall, the Zika epidemic is helping to reveal the placenta as one of the most understudied of organs.
"What our study suggests is not everyone is predisposed to having the virus replicate in the placenta, but the full meaning of this needs to be explored further."
Mehul S. Suthar PhD
Abstract
The present data demonstrate that primary HCs and CTBs isolated from full-term placentae are permissive to productive ZIKV infection by a contemporary strain currently circulating in the Americas. We also found that HCs respond to infection by triggering antiviral defense programs in the absence of overt cell death. In this limited study of five donors, we observed individual variability in kinetics and magnitude of virus replication, inflammation, and antiviral gene expression, likely reflecting differences in individual genetics (Querec et al., 2009, Thio, 2008). Though unlikely given the low number of cell passages PR 2015 has undergone, it is possible that minor cell culture adaptations or quasi-species may also be playing a role in donor-to-donor variability. These observations suggest that donors may have the capacity to restrict ZIKV at different stages of the viral replication cycle. This may also relate to observed differences in intrauterine transmission efficiency, where more susceptible HCs from a pregnant mother may support higher levels of virus replication and subsequent spread to the developing fetal nervous system. Additionally, it will be important in future studies to characterize when HCs and CTBs are most susceptible to ZIKV infection (i.e., first, second, or third trimester). Recent projections from the CDC based on data from Brazil indicate that virus infection during the first trimester or early in the second trimester of pregnancy is temporally associated with the observed increase in infants born with microcephaly (Reefhuis et al., 2016).
This work was funded in part by the National Institutes of Health, Children's Healthcare of Atlanta, Emory Vaccine Center, the Georgia Research Alliance, the Multi-Center NICHD International Maternal Pediatric Adolescent AIDS Clinical Trials Network, and the Center for AIDS Research at Emory University.
Cell Host & Microbe, Quicke and Bowen et al.: "Zika virus infects human placental macrophages" http://www.cell.com/cell-host-microbe/fulltext/S1931-3128(16)30211-6
Cell Press Statement on Data Sharing in Public Health Emergencies
The Cell Press family of journals is committed to ensuring that the global response to public health emergencies is informed by the best available research evidence and data, and as such, we will make all content concerning the Zika virus free to access. We will work in partnership with reviewers to fast-track review all submissions concerning Zika. We will adapt the editorial criteria that we apply to Zika submissions by asking reviewers to evaluate only if the research methods are sound and support the conclusions and if the work will contribute in some way toward resolving the immediate challenges. We will expedite publication of papers that meet these two criteria.
Cell Host & Microbe (@cellhostmicrobe), published by Cell Press, is a monthly journal that publishes novel findings and translational studies related to microbes (which include bacteria, fungi, parasites, and viruses). The unifying theme is the integrated study of microbes in conjunction and communication with each other, their host, and the cellular environment they inhabit. Visit: http://www.cell.com/cell-host-microbe. To receive Cell Press media alerts, contact press@cell.com.
Return to top of page
|
|
|
Jun 15, 2016 Fetal Timeline Maternal Timeline News News Archive
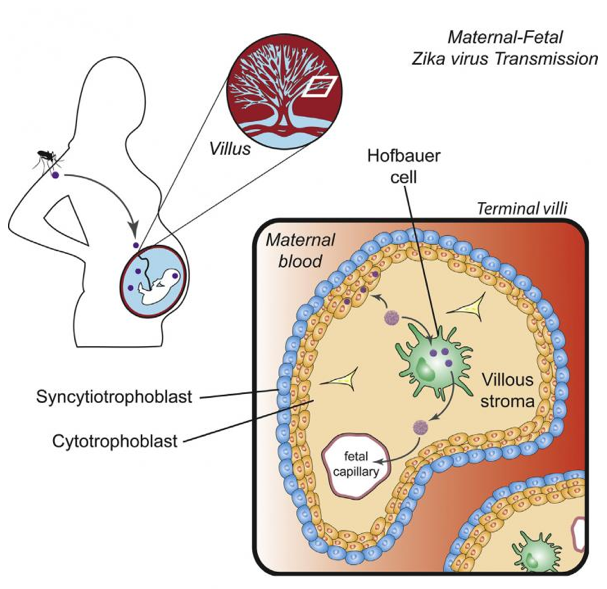
The findings of Quicke et al., demonstrate that a Caribbean ZIKA strain infects
and replicates in primary human placental macrophages and cytotrophoblasts,
suggesting a route ZIKA uses to cross the placental barrier.
Image Credit: Quicke and Bowen et al./Cell Host & Microbe 2016
|



