|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive Mar 19, 2015
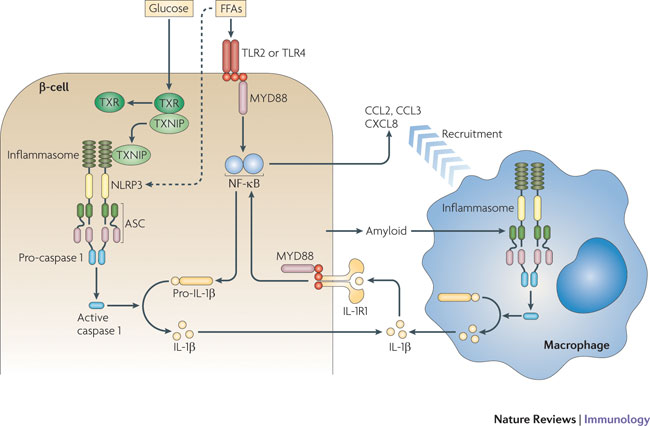
Diagram of signals being sent between a macrophage cell and a B-cell in an immune response.
Image Credit: Nature Immunology |
|
|
|
|
|
How dieting and fasting turn off inflammation
Researchers have found a compound produced, when dieting or fasting, that can block inflammation as seen in such disorders as type 2 diabetes, atherosclerosis, and Alzheimer's.
In a study published in Nature Medicine, Yale University researchers describe how the compound — β-hydroxybutyrate (BHB) — inhibits NLRP3, one protein in a set of proteins that make up the inflammasome which drives our inflammatory response. Knowing how to control infammation could help control numerous disorders such as autoimmune diseases, type 2 diabetes, Alzheimer's, atherosclerosis, and autoinflammatory disorders.
"Endogenous metabolites° — like BHB — block NLRP3 inflammasome which could be relevant against many inflammatory diseases, including those [caused] from mutations in the NLRP3 gene."
Vishwa Deep Dixit PhD, professor, Section of Comparative Medicine and Program on Integrative Cell Signaling and Neurobiology of Metabolism, Yale School of Medicine.
°molecular products cast off by chemical reactions within an organism
Though it was well known that fasting and calorie restriction reduces inflammation, it was unclear how immune cells adapted. Now by working with mice created carrying human immune cells, Vishwa Deep Dixit and colleagues could focus on how macrophages — immune cells — respond when exposed to the ketone bodies, water-soluble molecules produced by the liver from fatty acids following periods of fasting.
BHB, along with acetoacetate (AcAc), supports our survival through states of low energy, such as fasting. These molecules can create alternative sources of ATP — the high-energy molecule that fuels just about all of our metabolic activity.
BHB increases during fasting, high-intensity exercise, caloric restriction, or when eating a low-carbohydrate ketogenic diet, a diet that forces the body to burn fats for energy instead of carbs.
So, the team introduced BHB to mice modelling inflammatory diseases caused by NLP3. NLP3 is a molecular dirived from the NLRP1 gene. When the NLRP1 gene is over expressed it stimulates apotosis or programmed cell death. The prescence of BHB reduced inflammation in the mice. But, it was also observed that inflammation could be reduced when mice were put on a ketogenic diet as well, as a low-carbohydrate diet also elevates BHB in the blood.
"Our results suggest that endogenous metabolites like BHB (produced in response to low-carb dieting, fasting, or high-intensity exercise) can lower NLRP3 inflammasome."
Vishwa Deep Dixit PhD
Abstract
The ketone bodies β-hydroxybutyrate (BHB) and acetoacetate (AcAc) support mammalian survival during states of energy deficit by serving as alternative sources of ATP1. BHB levels are elevated by starvation, caloric restriction, high-intensity exercise, or the low-carbohydrate ketogenic diet2. Prolonged fasting reduces inflammation; however, the impact that ketones and other alternative metabolic fuels produced during energy deficits have on the innate immune response is unknown2, 3, 4, 5, 6. We report that BHB, but neither AcAc nor the structurally related short-chain fatty acids butyrate and acetate, suppresses activation of the NLRP3 inflammasome in response to urate crystals, ATP and lipotoxic fatty acids. BHB did not inhibit caspase-1 activation in response to pathogens that activate the NLR family, CARD domain containing 4 (NLRC4) or absent in melanoma 2 (AIM2) inflammasome and did not affect non-canonical caspase-11, inflammasome activation. Mechanistically, BHB inhibits the NLRP3 inflammasome by preventing K+ efflux and reducing ASC oligomerization and speck formation. The inhibitory effects of BHB on NLRP3 are not dependent on chirality or starvation-regulated mechanisms like AMP-activated protein kinase (AMPK), reactive oxygen species (ROS), autophagy or glycolytic inhibition. BHB blocks the NLRP3 inflammasome without undergoing oxidation in the TCA cycle, and independently of uncoupling protein-2 (UCP2), sirtuin-2 (SIRT2), the G protein–coupled receptor GPR109A or hydrocaboxylic acid receptor 2 (HCAR2). BHB reduces NLRP3 inflammasome–mediated interleukin (IL)-1β and IL-18 production in human monocytes. In vivo, BHB or a ketogenic diet attenuates caspase-1 activation and IL-1β secretion in mouse models of NLRP3-mediated diseases such as Muckle–Wells syndrome, familial cold autoinflammatory syndrome and urate crystal–induced peritonitis. Our findings suggest that the anti-inflammatory effects of caloric restriction or ketogenic diets may be linked to BHB-mediated inhibition of the NLRP3 inflammasome.
Other authors on the study include Yun-Hee Youm, Kim Y. Nguyen, Ryan W Grant, Emily L. Goldberg, Monica Bodogai, Dongin Kim, Dominic D'Agostino, Noah Planavsky, Christopher Lupfer, Thirumala D Kanneganti, Seokwon Kang,?Tamas L. Horvath, Tarek M. Fahmy, Peter A. Crawford, Arya Biragyn, and Emad Alnemri.
The research was funded in part by National Institutes of Health grants AI105097, AGO43608, AG031797, and DK090556.
Return to top of page
|
