|
Sperm pass on information about dad's weight
Turns out dads are also eating for two. A study reveals that a man's weight changes the heritable information in his sperm — and passes that trait on to his children.
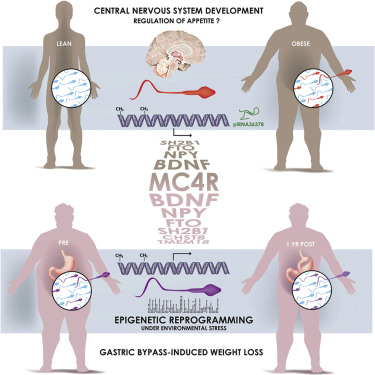
Sperm cells in lean and obese men have different epigenetic — or non-DNA — marks. They are most notable in genes that control appetite.
Research which included 13 lean men and 10 obese men, offers one explanation for why children of obese fathers are also predisposed to being obese. The work was
published December 3, 2015 in Cell Metabolism.
In one phase of the study, investigators tracked 6 men undergoing weight-loss surgery to see if it might affect sperm. On average, 5,000 structural changes in sperm cell DNA were observed before, at the moment of, and one year later following surgery. Although more needs to be learned about these differences - including the effects on offspring - early evidence reveals sperm pass on information about the father's current health.
"Our research could lead to changing behavior, particularly pre-conception behavior of fathers.
"It's common knowledge that when a woman is pregnant she should take care of herself — not drink alcohol, stay away from pollutants, etc.
"But if the implications of our study hold true, then modifying behavior should be directed towards men, too."
Romain Barrès PhD, Associate Professor, University of Copenhagen and senior author.
Barrès was inspired by a 2005 study of people living in a small Swedish village during periods of famine.
The nutritional stress on the grandparents was passed down to their grandchildren who developed cardiometabolic diseases. Epigenetic marks (the methylation tags on histone proteins) affected by periods of starvation resulted in structural changes to molecules called small RNAs.
Epigenetic marks can influence how genes are expressed ["read"], which has been shown to affect the health of offspring in insects and rodents as well.
In his study, Barrès and colleagues followed and compared specific epigenetic marks in the ejaculate of lean and obese men — sperm being much easier to obtain than eggs.
While no differences were seen in the histone proteins that tightly wrap DNA to fit within a cell, there were variations in the mens' small RNAs. Small RNAs are not translated into proteins. Instead, these 20–30-nucleotide sequences regulate various biological processes, often by interfering with the translation of messenger RNA - Nature. Messenger RNA (mRNA) specifies what amino acid sequence of proteins should be expresessed by the gene.
Variations were also seen in the methylation of genes associated with brain development and appetite.
The next question was whether these differences were by products of obesity or lifestyle. This led to the examination of male patients following bariatric surgery to see if it affects sperm epigenetics, and the discovery that weight gain was the main result in children.
There are likely evolutionary reasons why information from a father's weight are valuable to his offspring. Barrès theory is that in times of abundance, it is a programmed way to encourage children to eat more in order to store energy.
"It's only recently that obesity is not an advantage. Only decades ago, the ability to store energy was an advantage to resist infection and famine."
Romain Barrès PhD
To learn more about the epigenetic to offspring connection, the Barrès lab is now collaborating with a fertility clinic to study epigenetic differences in discarded embryos generated from the sperm of men with various degrees of body weight. (By law in Denmark, after five years, embryos must be discarded and can be used for research.)
Further comparative data will be taken from cord blood of children fathered by each of the men. But this aspect of the research will take some time to accumulate in order to produce a large cohort of participants.
"It is clear that these epigenetic changes happen in mice and rats. But, we also need to know if this happens in humans and whether this is a significant driver for changing inherited traits."
Romain Barrès PhD
Abstract Highlights
•Distinct sncRNA expression and DNA methylation profiles in sperm from obese humans
•Differentially methylated genes are related to brain function
•The spermatozoal epigenome is dynamically remodeled after bariatric surgery
•Differential methylation clusters with known SNPs of obesity
Summary
Obesity is a heritable disorder, with children of obese fathers at higher risk of developing obesity. Environmental factors epigenetically influence somatic tissues, but the contribution of these factors to the establishment of epigenetic patterns in human gametes is unknown. Here, we hypothesized that weight loss remodels the epigenetic signature of spermatozoa in human obesity. Comprehensive profiling of the epigenome of sperm from lean and obese men showed similar histone positioning, but small non-coding RNA expression and DNA methylation patterns were markedly different. In a separate cohort of morbidly obese men, surgery-induced weight loss was associated with a dramatic remodeling of sperm DNA methylation, notably at genetic locations implicated in the central control of appetite. Our data provide evidence that the epigenome of human spermatozoa dynamically changes under environmental pressure and offers insight into how obesity may propagate metabolic dysfunction to the next generation.
This work was supported by a grant from the Novo Nordisk Foundation - Endocrinology Research.
Cell Metabolism, Donkin and Versteyhe et al.: "Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans" http://dx.doi.org/10.1016/j.cmet.2015.11.004
Cell Metabolism (@Cell_Metabolism), published by Cell Press, is a monthly journal that publishes reports of novel results in metabolic biology, from molecular and cellular biology to translational studies. The journal aims to highlight work addressing the molecular mechanisms underlying physiology and homeostasis in health and disease. For more information, please visit http://www.cell.com/cell-metabolism. To receive media alerts for Cell Press journals, contact press@cell.com.
Return to top of page
|