|
|
A quick, simple way to generate neural crest cells
Research led by the School of Medicine at the University of California, Riverside (UC Riverside) provides a quick, simple and trackable way to generate neural crest cells.
Neural crest cells develop early, becomming many diverse cell types. They contribute to our peripheral nerves, melanocytes in our skin to protect us from UV light, and facial cells that contribute to muscle, bone, cartilage and teeth.
When neural crest biology fails, cleft lip/palate, Hirschsprung and Waardenburg syndromes, melanoma and neuroblastoma can result. So understanding these cells is crucial for diagnosis and treatment. But access to human embryonic cells is difficult. While generating human neural crest cells from human embryonic stem cells has progressed in the last 11 years, enormous limitations still exist. Some protocols use ingredients — such as blood serum — which can contain unknown components and complicate identification of the molecules forming.
Even the fastest of these protocols takes 12 days to convert human
embryonic stem cells into neural crest cells. Often the results provide low cell yields, making neural crest cells a time-consuming and technically challenging process.
"Our study provides a model to generate neural crest cells in just five days. Starting from human embryonic stem cells or induced pluripotent cells, we use a simple and well-defined medium with all ingredients known and accounted for," expains Martín I. García-Castro PhD, whose lab led the study. "Our cost-effective, efficient and fast protocol allows a better analysis of the relevant signals and molecules involved in the formation of these cells."
"Our results suggest that human neural crest cells can arise independently — and before the formation of mesoderm and neural ectoderm. Both of which had been thought to be critical in neural crest formation."
Martín I. García-Castro PhD, Department of Molecular, Cellular and Developmental Biology, Yale University, New Haven, Connecticut ; and School of Medicine, University of California Riverside, Riverside, California, USA
The work is
published in the journal Development, 2/1/16.
In the early embryo, the mesoderm is the middle layer of cells forming between the endoderm and the ectoderm (the outermost layer). In order to identify specific molecules and their role in neural crest formation, García-Castro's reinforces that Wingless WNT molecular pathways contribute proteins to regulate cell-to-cell interactions — even helping adult tissues remain stable and constant. While the fibroblast growth factor, or FGF protein family, is thought to control branching, bone morphogenetic proteins (BMPs) induce bone and cartilage formation.
García-Castro emphasized that the proper function of neural crest cells is essential for human development and health. WNT signals cell to cell communication, inducing change in nearby cells and altering their behavior.
"Our work provides strong evidence for the critical and initiating role of WNT signals in neural crest cell formation, later contributing to FGF and BMP pathways.
"The study of these cells is essential to improve clinical efforts to diagnose, manage, and perhaps prevent diseases and conditions linked to them. Our lab has already launched efforts towards facial clefts - lip and or palate - and melanoma, and we hope to make substantial progress in both areas thanks to this novel protocol."
Martín I. García-Castro PhD
Abstract
Neural crest (NC) cells arise early in vertebrate development, migrate extensively and contribute to a diverse array of ectodermal and mesenchymal derivatives. Previous models of NC formation suggested derivation from neuralized ectoderm, via meso-ectodermal, or neural-non-neural ectoderm interactions. Recent studies using bird and amphibian embryos suggest an earlier origin of NC, independent of neural and mesodermal tissues. Here, we set out to generate a model in which to decipher signaling and tissue interactions involved in human NC induction. Our novel human embryonic stem cell (ESC)-based model yields high proportions of multipotent NC cells (expressing SOX10, PAX7 and TFAP2A) in 5 days. We demonstrate a crucial role for WNT/β-catenin signaling in launching NC development, while blocking placodal and surface ectoderm fates. We provide evidence of the delicate temporal effects of BMP and FGF signaling, and find that NC development is separable from neural and/or mesodermal contributions. We further substantiate the notion of a neural-independent origin of NC through PAX6 expression and knockdown studies. Finally, we identify a novel pre-neural border state characterized by early WNT/β-catenin signaling targets that displays distinct responses to BMP and FGF signaling from the traditional neural border genes. In summary, our work provides a fast and efficient protocol for human NC differentiation under signaling constraints similar to those identified in vivo in model organisms, and strengthens a framework for neural crest ontogeny that is separable from neural and mesodermal fates.
Competing interests
The authors declare no competing or financial interests.
Supplementary information available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.130849/-/DC1
García-Castro came to UC Riverside in November 2014. His coauthors on the research paper are Alan W. Leung (first author of the research paper, currently at Yale University, Conn.) and Barbara Murdoch (currently at Eastern Connecticut State University), both of whom are former members in his lab; and Ahmed F. Salem, Maneeshi S. Prasad, and Gustavo A. Gomez at UC Riverside.
Funding
This work is supported by funding from the National Institutes of Health National Institute of Dental and Craniofacial Research [2R01DE017914]; and Connecticut Innovations [14-SCB-YALE11]. Deposited in PMC for release after 12 months.
The University of California, Riverside (http://www.ucr.edu) is a doctoral research university, a living laboratory for groundbreaking exploration of issues critical to Inland Southern California, the state and communities around the world. Reflecting California's diverse culture, UCR's enrollment has exceeded 21,000 students. The campus opened a medical school in 2013 and has reached the heart of the Coachella Valley by way of the UCR Palm Desert Center. The campus has an annual statewide economic impact of more than $1 billion. A broadcast studio with fiber cable to the AT&T Hollywood hub is available for live or taped interviews. UCR also has ISDN for radio interviews. To learn more, call (951) UCR-NEWS.
Return to top of page
|
|
|
Feb 10, 2016 Fetal Timeline Maternal Timeline News News Archive
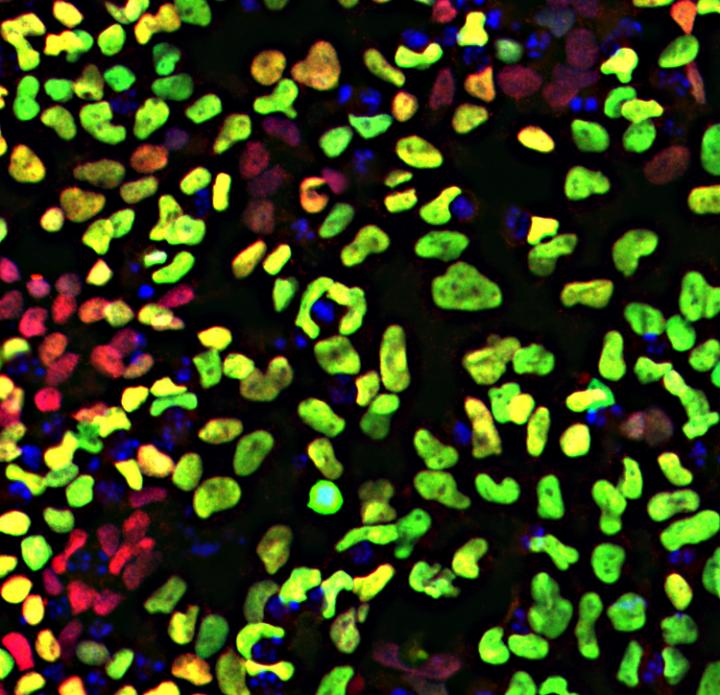
Human embryonic stem cells display neural crest cells after only five days in culture,
when induced with WNT. Transcription factors SOX10 and PAX7 are green and red.
Image Credit: García-Castro lab, UC Riverside
|
|
|
|



